Management of HER2-Positive Metastatic Breast Cancer: Standard and New Approaches
 ABSTRACT
ABSTRACT
Over expression of the human epidermal growth factor receptor 2 (HER2, ErbB2) is a negative prognostic factor associated with increased recurrence and shorter survival in breast cancer. Trastuzumab is the first modern targeted therapy specifically designed for the treatment of HER2-positive breast cancer. Although trastuzumab-based therapy has changed the treatment paradigm of HER2-positive metastatic breast cancer, most patients eventually develop progressive disease. This review discusses the advances being made in the management of HER2-positive metastatic breast cancer, with a special focus on trastuzumab resistance, treatments after progression on trastuzumab, and newer biological agents that may provide further therapeutic options for these patients.
Key words: HER2 human epidermal growth factor receptor 2, MBC metastatic breast cancer, Trastuzumab, Neratinib, T-DM1 Trastuzumab DM1
 INTRODUCTION
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2, ErbB2) is overexpressed in 20% to 30% of invasive breast cancer (1, 2). Members of the ErbB growth factor receptor family (ErbB1-ErbB4) share a common molecular structure that includes an extracellular domain (ECD), a transmembrane domain, and an intracellular tyrosine kinase domain, with the exception of HER3 which does not have the latter (3). Ligand-induced dimerization of these receptors results in activation of subcellular signal transduction pathways such as Ras/Raf/mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway. This in turn regulates various physiological process including cell growth, proliferation, and apoptosis (4). The amplification of the HER2 gene leads to HER2 receptor overexpression and confers a more aggressive clinical behavior compared with hormone receptor-positive and -negative disease. Several agents have been developed to target the HER2 receptors and include monoclonal antibodies and small molecule tyrosine kinase inhibitors. Trastuzumab (Herceptin) is a humanized monoclonal antibody that binds to the ECD of the HER2 receptor. It exerts its antitumor activity through a variety of proposed mechanisms, including the disruption of functional dimers involving HER2, reduction of the ECD shedding, and recruitment of immune effector cells, which results in antibody-dependent cell-mediated cytotoxicity (ADCC) (5, 6). The combination of trastuzumab with certain cytotoxic agents and hormonal agents has substantially reversed the negative prognostic impact of HER2 amplification (7–9). This review discusses the advances in the management of HER2-positive metastatic breast cancer (MBC), with a special focus on trastuzumab resistance, treatments after progression on trastuzumab, and other newer biological agents.
 HORMONE RECEPTOR- POSITIVE MBC
HORMONE RECEPTOR- POSITIVE MBC
Because of its more favorable side effect profile, endocrine therapy is the preferred first-line therapy for endocrine-responsive MBC unless there is rapidly progressive visceral metastasis. The combination of endocrine therapy and HER2-targeted therapy for HER2-overexpressing tumors has improved efficacy compared with endocrine therapy alone (7, 8). The TAnDEM trial randomly assigned 208 postmenopausal patients with HER2-positive, estrogen receptor/progesterone receptor (ER/PR)-positive MBC to anastrozole alone or anastrozole with trastuzumab (4 mg/kg on week 1 followed by 2 mg/kg weekly) until disease progression (7). The benefits of the combined therapy included a better overall response rate (RR) (20% vs 7%, P = .018) and progression-free survival (PFS) (4.8 months vs 2.4 months, P = .0016), though this significance may not be clinically meaningful. Overall survival was 28.5 months versus 23.9 months (P = .325). Cardiotoxicity was observed in 14 out of 103 patients in the combination group compared with 2 out of 104 patients in the anastrozole only group.
A similar trial, EGF30008, explored the benefit of letrozole with and without lapatinib (Tykerb), a tyrosine kinase inhibitor of both EGFR (ErbB1) and HER2 (8). This study was conducted as first line treatment in 1,286 postmenopausal patients with hormone receptor-positive and both HER2-positive and HER2-negative tumors. Median PFS in the HER2-positive population was significantly increased from 3.0 months in the letrozole group to 8.2 months in the lapatinib plus letrozole group (hazard ratio [HR] = 0.71, P = .019) with no difference in overall survival.
These two studies provide evidence that HER2-positive tumors are less responsive to endocrine therapy, with a PFS of 2.4 to 3 months with endocrine therapy alone. This also suggests that HER2-targeted therapy with endocrine therapy or chemotherapy is a better option for most patients with HER2-positive and hormone-responsive MBC compared with endocrine therapy alone. However, in patients with indolent disease, it is reasonable to initially start with endocrine and HER2-targeted therapy and then switch to chemotherapy if there is no efficacy. As discussed later, preclinical studies have established that HER2 amplification leads to endocrine resistance.
 TRASTUZUMAB COMBINED WITH ANTHRACYCLINES
TRASTUZUMAB COMBINED WITH ANTHRACYCLINES
The benefit of adding trastuzumab to anthracycline-based chemotherapy in the first-line setting was tested in a pivotal multicenter trial of 469 women with HER2-positive MBC (9). The addition of trastuzumab to chemotherapy was associated with a significantly longer time to progression (7.4 vs 4.6 months, P < .001), higher RR (50% vs 32%, P < .001), and longer median overall survival (25 vs 20 months, P = .046). Trastuzumab was shown to increase clinical benefit when added to first-line chemotherapy for patients with MBC who overexpress HER2.
The overall RRs were 56% for trastuzumab plus doxorubicin and cyclophosphamide (AC) and 42% for AC alone (P = .02). Therefore, trastuzumab plus concomitant anthracycline demonstrated significant clinical activity. However, cardiotoxicity was also more pronounced with the combination of AC plus trastuzumab. Symptomatic cardiac dysfunction (NHYA class III or IV) developed in 27% of patients in the combined group, compared with 8% treated with AC alone. These results led to the general recommendation that concomitant anthracyclines and trastuzumab should be avoided.
A recent prospective phase I/II trial called HERCULES evaluated the cardiac effects of trastuzumab plus epirubicin and cyclophosphamide (10). Dose-limiting cardiotoxicity was seen in 5% of patients receiving epirubicin at 90 mg/m2 and in 1.7% of patients treated with 60 mg/m2 of epirubicin. These findings were more in line with the trial by Marty et al. (11), where symptomatic cardiac toxicity was noted in 1% of patients treated with a non-anthracycline regimen of trastuzumab and docetaxel in the first-line metastatic setting. The tumor RR was 60% for the group treated with 90 mg/m2 of epirubicin and was 57% for the group that received epirubicin at 60 mg/m2 (10). This trial showed that the combination of trastuzumab with epirubicin and cyclophosphamide is a feasible and potentially an effective regimen for patients with HER2-positive MBC.
 TRASTUZUMAB COMBINED WITH TAXANES
TRASTUZUMAB COMBINED WITH TAXANES
In the pivotal trial by Slamon et al., trastuzumab and paclitaxel were compared with paclitaxel alone in patients with metastatic HER2-positive breast cancer. The combined therapy was associated with a significantly higher RR, 38% versus 16% (P < .001) and a trend toward better median overall survival (9). Since then, other trials have also proven weekly paclitaxel plus trastuzumab to be a particularly well-tolerated and an active regimen (12, 13). The clinical question is whether to use taxanes in combination or in sequence after progression on trastuzumab? This was addressed in the phase II HERTAX trial, in which 101 women with HER2-positive MBC previously untreated with chemotherapy were randomly assigned to combined therapy with trastuzumab plus docetaxel or trastuzumab monotherapy followed by docetaxel alone at progression (14). The primary endpoint was PFS. In a preliminary report, no significant benefit of combined therapy to sequential therapy was seen in terms of PFS (9.4 vs 10.8 months), and the difference in median overall survival was not significant. The combination of a taxane and trastuzumab is now considered to be a standard first-line therapy for women with hormone receptor-negative or hormone-refractory HER2-overexpressing MBC, particularly if the disease is rapidly progressing. However, serial administration of trastuzumab alone followed by taxane at disease progression is also an acceptable alternative for relatively asymptomatic disease (15). Select clinical trials of taxanes and trastuzumab as first-line therapy for HER2-positive MBC are listed in Table 1 (9,11,14,16,17).
 TREATMENT WITH TRASTUZUMAB BEYOND PROGRESSION
TREATMENT WITH TRASTUZUMAB BEYOND PROGRESSION
An important clinical question is whether trastuzumab should be continued after progression on a trastuzumab-containing regimen. After disease progression on a trastuzumab-based regimen as first-line treatment, patients either continue with trastuzumab plus chemotherapy or the regimen is changed to a nontrastuzumab-based one. An extension of the pivotal trial by Slamon et al. (18) showed the clinical benefit of trastuzumab beyond progression in HER2-positive MBC. In this extension trial, patients with progression were allowed to receive trastuzumab with another chemotherapy, and the choice of which was left to the discretion of the treating physician. In patients who had previously received trastuzumab, there was an 11% objective RR, and the median duration of response was 6.7 months. Trastuzumab was well tolerated in this study and cardiac dysfunction was uncommon, occurring in approximately 2% of the patients who were previously treated with trastuzumab. These findings suggest that trastuzumab continues to demonstrate clinical activity in the treatment of HER2-positive MBC beyond disease progression.
TABLE 1
Select trials of trastuzumab and taxanes in the first-line setting for HER2-positive metastatic breast cancer
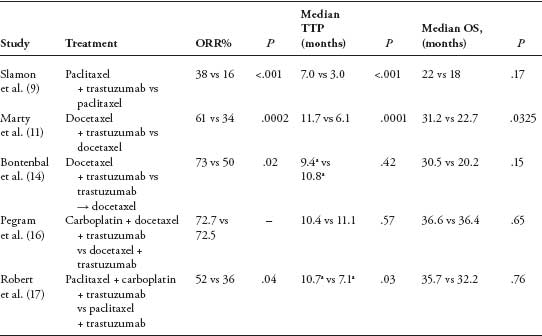
a Represents progression-free survival.
Another trial, the GBG-26/BIG 03–05 trial, randomly assigned 156 women with MBC who progressed on trastuzumab to capecitabine at 2,500 mg/m2 daily for 14 days on an every 21-day cycle alone or in combination with ongoing trastuzumab therapy given at 6 mg/kg every 3 weeks (19). Median overall survival was 25.5 months for the combination group and 20.4 months for the capecitabine group (HR = 0.76, P = .26). Although grade 3 and 4 toxicities are appreciable in both groups, the continuation of trastuzumab was not associated with increased toxicity. Therefore, continuing trastuzumab beyond progression appears to improve the efficacy of second-line capecitabine treatment.
Currently, there is a paucity of data addressing this issue, and in clinical practice trastuzumab is continued even after disease progression. Prospective randomized trials were closed secondary to poor accrual, and this is due to the reluctance of the physician and the patient to stop trastuzumab after evidence of disease progression. Currently, a phase III trial, THOR (Trastuzumab Halted or Retained), is ongoing to answer this question (20). This randomized, open-label study is designed to compare PFS in patients with HER2-positive MBC who continue or discontinue trastuzumab after progression on first-line chemotherapy with second line chemotherapy.
 MECHANISM OF RESISTANCE TO TRASTUZUMAB
MECHANISM OF RESISTANCE TO TRASTUZUMAB
The aforementioned studies show that trastuzumab has significant efficacy with other treatments in the first- and second-line setting for HER2-positive MBC. However, the overall primary resistance to trastuzumab is as high as 88%, and majority of patients who develop an initial positive response develop resistance to trastuzumab within a year (21). There are several proposed mechanisms for resistance to trastuzumab. One of these proposed mechanisms is the overexpression of MUC4, a member of the glycoprotein family, in breast cancer cells. It has been suggested that MUC4 augments the progression of cancer by blocking immune recognition of tumor cells (22). Another proposed mechanism is the shedding of the ECD of the HER2 receptor leaving behind the truncated form of the receptor, p95HER2, which retains its kinase activity, but cannot bind to trastuzumab. In an analysis of 46 patients with HER2-positive MBC, p95HER2 expression in tumors was associated with clinical resistance to trastuzumab (23). Alternative HER2-targeted therapy such as lapatinib, which targets the intracellular kinase domain of the HER2 receptor, might be an effective treatment option for patients who have resistance to trastuzumab secondary to overexpression of p95HER2.
Mutations of the PI3K/Akt signaling pathway have also been implicated in resistance to trastuzumab. Nagata and colleagues demonstrated that activation of phosphatase and tensin analogue (PTEN) contributes to the antitumor activity of trastuzumab and reduction of PTEN levels in breast cancer cells using antisense oligonucleotides which confers trastuzumab resistance both in vitro and in vivo (24). This study also showed that antagonists to PI3K, a primary downstream component of ErbB2 signaling, can also reverse trastuzumab resistance. Cross talk or heterodimerization between ErbB receptors that do not have HER2, such as ErbB1 homodimers or ErbB1/ErbB3 heterodimers, may lead to downstream signaling and can circumvent HER2-targeted inhibitors (25). Another transmembrane tyrosine kinase receptor, the insulin-like growth factor receptor 1 (IGF-1R), also can cause stimulation of cellular proliferation and suppression of apoptosis independent of the HER2 receptor (26). There is growing evidence that HER2 signaling is likely a mechanism for resistance to endocrine therapy in breast cancer (27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


