Classification
Stages/Scores
Parameters
Survival/Stage (%)
Hepatitis (%)
Study area of origin
Tumor characteristics
Liver function
Health status
Hepatitis B
Hepatitis C
Both
Okuda
I, II, III
</>50 % liver involvement
Bilirubin, Albumin, Ascites
——
I: 47
Not reported
II: 51
5 yr
Japan
III: 67
French
A: 0 pts
Portal invasion
+ AFP
Bilirubin
and alkaline phosphatase
Karnofsky
Index
A: 79
B: 1–5 pts
B: 31
1 yr
30
France
C: ≥6 pts
C: 4
CLIP
0, 1, 2, 3, 4, 5, 6
</>50 % liver involvement,
Portal invasion, AFP
CTP
——
0: 79
1: 64
2: 44
3: 19
3 yr
10
85.5
4.5
100
Italy
4: 13
5: 4
6: 0
BCLC
0: very early
Portal invasion, Metastasis, Morphology, Okuda
CTP + PTH + Bilirubin
PST
0:
A: 62
B: 28
3 yr
5
48
36.4
93
Spain
A: early
C: 28
B: intermediate
D: 10
C: advanced
D: end stage
CUPI
Low risk: ≤1
TNM, AFP
Ascites + Bilirubin + Alkaline phosphatase
Symptoms
Low risk: 47.9
Intermediate risk: 2–7
Intermediate risk: 17.5 1 yr
1 yr
82.3
77
Hong Kong
High risk: ≥8
High risk: 4.8
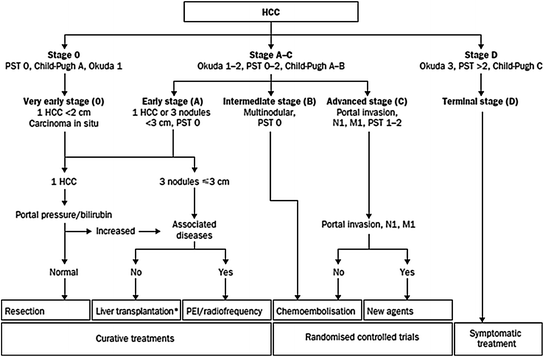
The BCLC staging system incorporates liver function (Child-Pugh score), tumor characteristics (the number and size of nodules, the presence or absence of vascular invasion, and the presence or absence of extrahepatic spread) and, unlike any of the other clinical staging systems, performance status. The five BCLC stages are as follows:
Stage 0, very early HCC: Tumor nodule <2 cm, and no manifestations of portal hypertension. Patients are typically good candidates for resection.
Early stage (A) HCC: A single nodule ≤5 cm or three nodules each up to 3 cm in diameter, compensated liver disease (Child-Pugh score A–B), and asymptomatic (PS score 0). Patients are suitable for potentially curative therapies (resection, transplantation, or ablation). Five-year survival rate is 50–75 %.
Intermediate-stage (B) HCC: Asymptomatic (PS score 0), with multinodular tumors but without vascular invasion or extrahepatic spread. Patients are eligible for locoregional therapy (TACE). Three-year survival rate may each 50 % even without treatment.
Advanced-stage (C) HCC: Symptomatic (PS score 1–2), or evidence of vascular invasion or extrahepatic spread. Patients are eligible for sorafenib. Three-year survival rate is approximately 10 %.
Terminal-stage (D) HCC: Severe cancer symptoms (PS score 3–4), or severely decompensated cirrhosis (Child-Pugh class C). Patients should receive treatment for symptoms only.
It is important to note that the BCLC treatment algorithm is based on a single institution experience. Moreover, it is fairly conservative with regard to the application of surgical therapy. Patients with larger singular tumors are not considered surgical candidates despite a growing experience with resection with acceptable outcomes in this group. Likewise, patient with multifocal disease who falls within the Milan criteria and comorbid conditions who may benefit from resection or transplantation are excluded from these therapies. Despite these concerns, the algorithm does provide a useful framework for the application of multimodality therapy for HCC.
Conspicuously absent from the BCLC staging system as well as all other current staging systems is the inclusion of molecular markers of disease biology. Work to establish such markers remains in a formative stage but some promising data have emerged. For example, Jonas and associates reported that increased tumor DNA aneuploidy, expressed as an index, is a more powerful prognostic indicator than tumor size, Milan Criteria, or vascular invasion in cirrhotic patients with HCC following liver transplantation [31]. Poon and associated reported that pretreatment serum VEGF levels independently predict overall and recurrence-free survival following radiofrequency ablation [32]. Kaseb and associates demonstrated a correlation between plasma IGF-1 levels and survival in patients with advanced HCC [33]. Further advances in molecular approaches are expected to decrease the marked heterogeneity noted in current staging systems available. With these refinements, the more selective and scientific application of current therapies may be possible.
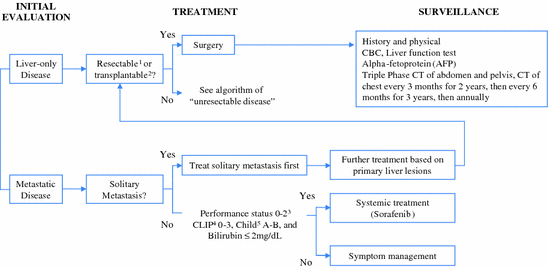
5 Selection of a Treatment Approach
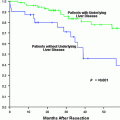
In general, patients with preserved liver function and small tumors are candidates for resection. Patients with preserved liver function and large tumors are usually candidates for resection as well, but location of the tumor(s) and the volume of the future liver remnant (FLR) are important factors in such cases. Patients with a small FLR or poor hepatic reserve have traditionally not been considered candidates for surgical resection although the indications for resection are expanding and remain controversial. The use of preoperative portal vein embolization can expand the number of potential candidates for resection.
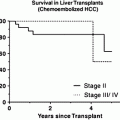
Patients with chronic liver disease and limited disease benefit from transplantation. Importantly, the indications for transplantation remain in a state of evolution with ongoing efforts to expand the Milan criteria, most notably at the University of Toronto and the University of California San Francisco [24, 34]. The limited availability of donor organs, however, necessitates the use of non-surgical local–regional therapies in such patients; either as a bridge to transplantation or as definitive therapy. Resection and transplantation also need not be viewed as mutually exclusive therapies. In appropriately selected patients, resection can be employed with transplantation reserved as a salvage therapy or for selected patients with higher risk features identified after pathologic assessment of the resected tumor (so called “de principe” transplantation), or as a bridge to transplantation [35–38].
Non-surgical local–regional options are extensive and include radiofrequency ablation, TACE, radioembolization, and radiotherapy. Radioembolization has been shown to forestall disease progression and may therefore prolong candidacy for transplantation [39]. Patients with disease burden that places them outside of transplant criteria (due to size or number of tumors) but without malignant portal vein thrombosis or extrahepatic metastatic disease, may also be candidates for radioembolization [39]. In some cases this modality can lead to downstaging of disease to within transplant criteria [40]. TACE is often the preferred treatment for palliation of unresectable HCC and is also employed as an adjunctive therapy to liver resection or as a bridge to OLT, as well as prior to or after radiofrequency ablation [3, 41–43]. Optimal candidates for TACE are those patients with unresectable lesions and preserved liver function without extrahepatic spread [44].
Advances in technology have also prompted a renewed interest in the use of radiation therapy as a potentially curative or palliative modality. Imaging-guided technologies allow for tumor targeting despite respiratory movement improving the precision of radiation delivery and theoretically limiting toxicity. Investigators have demonstrated excellent local control of unresectable HCC’s with both photon- and proton-based therapy [45–48]. Radiation has also been explored in combination with TACE with promising results [49–51]. Finally, systemic therapies are appropriate for patients with more extensive or disseminated disease. Sorafenib, which has been shown to forestall tumor progression and improve survival, is now the standard systemic agent in an advanced disease setting; however, promising novel systemic agents and combination therapies are emerging and can be offered in the context of a clinical trial [28].
Specific treatment goals for which a multidisciplinary approach pays the greatest dividends include: (1) conversion of unresectable to resectable disease in the patient with preserved liver function and performance status; (2) stabilization of disease in patients awaiting liver transplantation; and (3) palliation and prolonged survival in patients with advanced disease. The complexity of HCC treatment is increasing with the introduction of new therapies and expanding indications for established ones. Variability in the burden and characteristics of disease and availability of resource and expertise at a given institution necessitate treatment approaches that are both personalized and institution-specific. Moreover, these approaches must remain fluid enough to incorporate novel therapies as they emerge. At the University of Texas MD Anderson Cancer Center, the multidisciplinary team has established a treatment algorithm guiding the application of available therapies. This algorithm is included to exemplify our effort to provide treatment options for the full spectrum of patients with HCC (Figs. 2, 3).