Management of Dyspnea
Shalini Dalal
Gayatri Palat
Eduardo Bruera
Dyspnea has been defined as an uncomfortable awareness of breathing (1). Although everybody has experienced the sensation and has an intuitive understanding of this symptom, there is no universal agreement as to its definition. Dyspnea is a subjective sensation and cannot be defined by the physical abnormalities that accompany such an unpleasant subjective experience. For the purpose of this chapter, dyspnea is defined as an unpleasant sensation of difficult, labored breathing.
Dyspnea is a frequent and devastating symptom in patients with advanced cancer (2) and has been reported to occur in 21–79% of patients (3). A quality of life and survival prediction study in terminal cancer has shown that health care professionals should focus on physical health-related quality of life indicators, such as nausea and emesis, dyspnea, and weakness, to gather prognostic clues in patients with terminal cancer (4). There is evidence that good symptom control, even by experienced palliative care teams, is achieved less frequently for dyspnea than for other symptoms such as pain or nausea (5). In addition, limited research, and education are available on the adequate assessment and management of dyspnea in cancer patients.
The aim of this chapter is to review the pathophysiology, prevalence, assessment, and treatment of dyspnea in patients with cancer. Discussion of areas where future research should focus is also included.
Pathophysiology
Dyspnea is frequently associated with abnormalities in the mechanisms that regulate normal breathing. However, the actual expression of dyspnea by a patient results from a complex interaction between the abnormalities in breathing and the perception of those abnormalities in the central nervous system. The origins of dyspnea in different clinical settings can be traced to specific abnormalities. These are discussed in the following paragraphs.
Regulation of Breathing
Figure 25.1 summarizes the regulation of normal breathing. Respiration is integrated as a system with three main components, as discussed in the following sections.
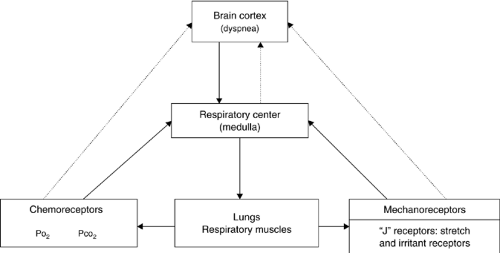 Figure 25.1. Regulation of normal breathing. PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide. |
Respiratory Center
The respiratory center is located in the medulla. Its neurons receive information from both central and peripheral chemoreceptors and peripheral mechanoreceptors. It also receives information from the cerebral cortex, which regulates voluntary breathing such as occurs during speaking and singing. Efferent neurons stimulate the diaphragm, the intercostal muscles, and the accessory muscles (6).
Receptors
The levels of oxygen and carbon dioxide in the blood stimulate chemoreceptors located centrally and peripherally. These chemoreceptors are capable of stimulating the respiratory center and increasing respiratory rate (7). Although strong debate continues on this subject, recent evidence suggests that chemoreceptors are probably also able to stimulate the brain cortex directly and cause dyspnea (7). An alternative explanation for the dyspnea caused by increases in PCO2 and decreases in PO2 is that chemoreceptors stimulate the respiratory center; increasing the respiratory effort, which stimulates mechanoreceptors capable of stimulating the brain cortex, resulting in the sensation of dyspnea. The mechanoreceptors are located primarily in the respiratory muscles and the lung. These receptors respond to either irritants or, more commonly, pulmonary stretch, including vascular congestion (6).
Respiratory Muscles
The respiratory muscles promote gas exchange. Changes in the PO2 and PCO2 are detected by the chemoreceptors. Changes in the tension within the abdominal wall and the lung are detected by the mechanoreceptors. This information is fed back to the respiratory center. Sensory receptors are found inside the respiratory muscles, including the intercostal, sternomastoid, and diaphragm. The balance between the contractual activity and stimulation of the sensory receptors is of great importance in the type of input provided to the respiratory center and the cortex.
Although the three aforementioned factors are the main elements in the regulation of breathing, the actual sensation of dyspnea is a result of cortical stimulation. The sensation of dyspnea has been related to the activation of mechanoreceptors in the respiratory muscles and lung (8). Elegant research has shown that both in normal volunteers and patients, with different stimuli capable of stimulating mechanoreceptors are able to produce dyspnea even in the absence of increased respiratory activity (6). In addition, two other possible mechanisms of dyspnea have been proposed. On one hand, the previously discussed role of chemoreceptor stimulation, on the other hand, some authors have proposed a role for the respiratory center as a potential cause of dyspnea by direct ascending cortical stimulation (7, 9).
Production of Dyspnea
Dyspnea is produced by physical and biochemical abnormalities (Fig. 25.2). The perception is then modulated by anxiety, depression, or administration of opioids. Somatization and cultural factors further influence a patient’s expression of dyspnea.
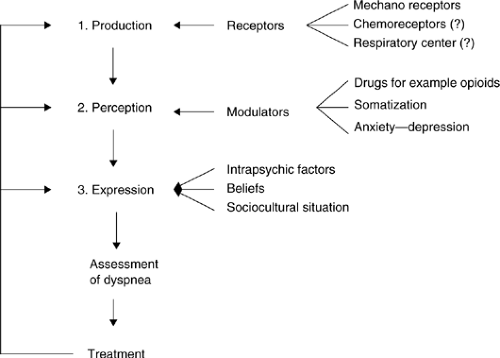 Figure 25.2. Stages in the production of dyspnea. ?, hypothesized/suspected but not absolutely clear. |
A number of researchers have found great variability in the expression of dyspnea among patients with similar levels of functional abnormalities. Among patients with asthma, approximately 15% did not report dyspnea despite severe air-flow obstruction (forced expiratory volume in 1 second (FEV1) of < 15% of the predicted) (10). Similarly, among patients with chronic obstructive pulmonary disease (COPD), the complaint of dyspnea was not well correlated with abnormalities in the pulmonary function tests (11). Among these patients who were defined as having disproportionate dyspnea (complaint of dyspnea in the presence of a mean FEV1 of 1.8 L), almost all patients were considered to have a psychiatric diagnosis (mostly anxiety and depression) (11). Anxiety has been shown to be an independent correlate of the intensity of dyspnea in patients with cancer (12). This association is not entirely clear as anxiety may contribute to dyspnea but may also arise from its presence.
These studies suggest that some patients have modulators that either amplify or decrease the intensity of the symptom that is perceived at the cortical level. Patients receiving drugs such as opioids for pain can perceive significantly less dyspnea (2).
Finally, the expression of a certain symptom may be influenced by cultural factors, the belief about the mechanism for the symptom, and other factors such as somatization (13). Because neither the production nor the perception of dyspnea can be measured at present, the entire assessment is based on the patient’s expression. This issue is discussed in the following section.
Clinical Situations Associated with Dyspnea
Dyspnea can result from three main pathophysiologic abnormalities (6):
An increase in respiratory effort to overcome a certain load (e.g., obstructive or restrictive lung disease, pleural effusion)
An increase in the proportion of respiratory muscle required to maintain a normal workload (e.g., neuromuscular weakness, cancer cachexia)
An increase in ventilatory requirements (hypoxemia, hypercapnia, metabolic acidosis, anemia, etc.)
In many patients with cancer, different proportions of the three abnormalities may coexist, thereby making the pathophysiologic interpretation of the intensity of dyspnea more complex.
Role of Respiratory Muscles
During recent years a number of authors have found that respiratory muscle weakness has an important role in the dyspnea associated with a number of chronic conditions. Palange et al. (14) found that malnutrition significantly affected the muscle aerobic capacity and exercise tolerance in patients with COPD. They suggested that high wasted ventilation might be responsible for the weight loss. Diaphragmatic fatigue has been associated with dyspnea in patients with COPD (15). In chronically malnourished patients without pulmonary disease, malnutrition reduces respiratory muscle strength and maximal voluntary ventilation. Therefore, malnutrition might impair the respiratory muscle capacity to handle increased ventilatory loads in cardiopulmonary disease (16). In normal volunteers, the sensation of dyspnea has been correlated with respiratory muscle fatigue (17).
A study of patients with cancer determined that the maximal inspiratory pressure, a reliable functional test of the strength of the diaphragm and other respiratory muscles, is severely impaired in patients with cancer and dyspnea (18). Maximal inspiratory pressure has subsequently been found to be an independent correlate of the intensity of dyspnea in a subgroup of patients with advanced cancer and with moderate to severe dyspnea (12).
Prevalence of Dyspnea
The large variation in reported prevalence of dyspnea (21–79%) is a result of the different natures of patient populations reported by different authors and the lack of a general consensus on the assessment methods for identifying and quantifying the presence and intensity of dyspnea.
Higginson and McCarthy (5) conducted a prospective study in 86 consecutive patients with advanced cancer referred to a Community Palliative Care Service. Eighteen patients (21%) reported dyspnea as their main symptom before death. The symptom assessment scores for patients with dyspnea showed no change overtime, as compared with a significant decrease in the intensity of pain reported by this same patient population.
In the National Hospice Study, Reuben et al. (19) reported a high prevalence of dyspnea, with 70% of 1754 patients experiencing this symptom sometime during the last 6 weeks of life. 27.5% of patients reported dyspnea to be present all the time and more than 28% of patients rated the severity of their symptoms as moderate or worse during the self-report assessment. Although 33% of patients had a diagnosis of primary or metastatic lung cancer, 24% of patients with dyspnea did not have any known lung or heart disease or evidence of pleural effusion. Grond et al. (20) reported a prevalence of dyspnea of 24% among 1635 patients with cancer referred to a pain clinic. Donnelly et al. (21) found dyspnea in 28% of 1000 patients referred for consultation to a palliative care service. Of those patients who reported dyspnea, 63% rated this symptom as moderate or severe.
Twycross and Lack (22) found a prevalence of 51% of dyspnea in 6677 patients admitted to a palliative care program. Muers (23) found breathlessness to be a presenting complaint for 60% of 289 patients with non–small cell lung cancer, half of whom described their shortness of breath as moderate or severe.
A number of authors have reported dyspnea in a significant percentage of patients with advanced cancer without intrathoracic malignancy. The National Hospice Study (19) found a frequency of 24% in patients with no known lung or heart disease. Cachexia occurs in more than 80% of patients with advanced cancer (24). In addition, asthenia, and electrophysiological abnormalities in muscle function are detected in a large proportion of patients with advanced cancer. A recent study of 222 patients with chronic congestive heart failure found that dyspnea was the exercise-limiting symptom in 160 and generalized fatigue in 62 patients. No significant differences were found between any of the cardiovascular parameters of these two groups. The authors concluded that both symptoms are “two sides of the same coin,” and that they express the same underlying pathophysiologic process (25). It is possible that in some patients with advanced cancer, dyspnea may be one clinical expression of the syndrome of overwhelming cachexia and asthenia that is highly prevalent in the comprehensive assessment of these patients, including frequent pulmonary function tests.
In summary, dyspnea appears to be a common symptom in patients with advanced cancer. It is reported more commonly in patients during the last weeks of life. Although it is more common among patients with lung cancer or pulmonary metastases, it is also frequent in patients with no demonstrable tumor involvement in the lung. Most patients who develop dyspnea tend to rate this symptom as one of their main problems.
Smoke is considered the major cause of lung cancer although other factors may be involved in its pathogenesis. In developing countries, wood, and other solid fuels (26) are used for cooking and heating. Exposure to biomass smoke has been associated with respiratory diseases such as chronic bronchitis, emphysema, and asthma (27).
Although the causes of dyspnea in cancer are more varied than the causes of dyspnea in COPD, many are similar, thereby providing the justification for recommending the best practice from COPD research to be used in lung cancer. Figure 25.3 summarizes the common causes of dyspnea in patients with cancer.
 Figure 25.3. Common cause of dyspnea in patients with advanced cancer. (COPD), chronic obstructive pulmonary disease |
Less common causes of dyspnea in patients with cancer include atelectasis, phrenic nerve palsy, tracheal obstruction, carcinomatous infiltration of the chest wall, abdominal distention, pneumothorax, and metabolic acidosis. The pathophysiology of dyspnea in most patients with cancer is complex. For example, a given patient may have an increase in respiratory effort necessary to overcome the presence of a large pleural effusion, in addition to an increase in the proportion of respiratory muscle required for breathing because of cachexia and increased ventilatory requirement resulting from severe anemia.
Assessment
Although most patients with cancer develop dyspnea as a progressive complication over days or weeks, some patients present with sudden onset of dyspnea as an acute medical
emergency. The management of the latter group always should be considered a medical emergency.
emergency. The management of the latter group always should be considered a medical emergency.
History
The cause of dyspnea can be determined in most patients by taking an adequate history and performing a physical examination. The intensity of dyspnea should be assessed using a validated system. In addition, descriptors of dyspnea may provide a clue as to the pathophysiologic basis of dyspnea.
Intensity of Dyspnea
Because dyspnea is a subjective symptom with multiple potential etiologies, objective findings such as tachypnea or oxygenation saturation levels may not adequately reflect the distress experienced by patients with dyspnea. The presence and intensity of dyspnea should be assessed using validated assessment tools with numerical, verbal analogue, or visual analog scales (28, 29). The intensity of dyspnea is included in some of the available supportive care tools, such as the Support Team Assessment Schedule (3) and the Edmonton Symptom Assessment System (29). Quality of life questionnaires, including the European Organisation for Research and Treatment in Cancer Quality-of-Life Core Questionnaire, have provided for a more detailed assessment of the intensity of dyspnea in certain modules (e.g., lung cancer) (30).
In addition, some specific dyspnea questionnaires such as the chronic respiratory questionnaire (CRQ) and the Medical Research Council Scale have been found to be useful in patients with COPD (31, 32), and with dyspnea from a variety of respiratory and cardiovascular origins, respectively (33, 34). The Borg Category Scale (35) was developed for rating perceived dyspnea during exercise.
One of the main problems associated with assessment of dyspnea is the variable intensity of this symptom according to the level of activity and during different times of the day. In patients with respiratory and cardiovascular diseases, one approach to this problem has been the performance of dyspnea-causing activities (such as progressive exercising on a treadmill or bicycle) to assess pharmacologic and nonpharmacologic interventions (36). However, most patients with cancer are too ill to participate in these tests. One potentially less invasive approach to dyspnea assessment is breath-holding (37, 38) and reading (39) to measure the limiting effects of breathlessness. Immediately after breath-holding, there is a period when no particular respiratory sensation is experienced (20–30 seconds in healthy subjects). This is followed by a second period in which there is progressive discomfort until breaking point. It has been found that training plays an important role in prolongation of total breath-holding time, but has little effect on the period of no respiratory sensation. In addition, the period of no respiratory sensation in patients with COPD is apparently shortened and measurement of this period can be useful in the study of the genesis of dyspnea (38). Breath-holding has not been prospectively validated for use as an assessment tool in cancer dyspnea.
A study (39) looked at a simple test involving reading of numbers in 30 patients with cancer and 30 controls. Patients read fewer numbers, and fewer numbers per breath than controls. Twelve of the 30 patients were unable to complete all five readings in all three tests due to tiredness. The test was found to have good repeatability, both within and between days, and was sensitive to improvement seen following drainage of pleural effusions in 13 patients.
In summary, a large number of scales are available for assessment of the intensity of dyspnea. They range from simple analog and numerical scales to more complex scales, including multiple items. Many of these scales have been adequately validated and are highly reproducible.
Descriptors of Dyspnea
In the case of pain, specific descriptors are associated with specific pathophysiologic syndromes (40). For example, a burning, or numb sensation has traditionally been associated with neuropathic pain. In many cases, the descriptor alone is enough to make a diagnosis and suggest the need for specific drug therapy. Simon et al. (41) attempted to associate specific descriptors with specific pathophysiology in 53 patients with dyspnea caused by a number of known causes. Patients were asked to choose descriptions of their sensation of breathlessness from a dyspnea questionnaire listing 19 descriptors. Cluster analysis then was used to identify natural groupings among those descriptors. Although descriptors, such
as “rapid” or “heavy,” were associated with exercise, “tight” was frequently associated with asthma, and “suffocating” was frequently associated with congestive heart failure. Mahler et al. (42) used a questionnaire with 15 items that described qualities of breathlessness in 218 patients who sought medical care for breathlessness. The authors concluded that patients with different cardiorespiratory conditions experience distinct qualities of breathlessness and that using a questionnaire containing descriptors of dyspnea might help to establish a specific diagnosis. However, a recent study has suggested that different ethnic groups use different words to describe breathlessness in the presence of air-flow obstruction (43). This requires further clarification. In addition, more research is needed to better characterize the quality of dyspnea associated with specific clinical conditions.
as “rapid” or “heavy,” were associated with exercise, “tight” was frequently associated with asthma, and “suffocating” was frequently associated with congestive heart failure. Mahler et al. (42) used a questionnaire with 15 items that described qualities of breathlessness in 218 patients who sought medical care for breathlessness. The authors concluded that patients with different cardiorespiratory conditions experience distinct qualities of breathlessness and that using a questionnaire containing descriptors of dyspnea might help to establish a specific diagnosis. However, a recent study has suggested that different ethnic groups use different words to describe breathlessness in the presence of air-flow obstruction (43). This requires further clarification. In addition, more research is needed to better characterize the quality of dyspnea associated with specific clinical conditions.
Physical Examination and Investigations
A general physical examination with focus on the cardiac and respiratory systems is essential. A chest radiograph, digital oximetry, and simple blood tests can help in determining its cause. Pulmonary function tests can be particularly useful in the assessment of obstructive and restrictive pulmonary disorders as well as neuromuscular weakness. These tests can be performed repeatedly at the bedside and are useful in assessing the response to different therapies, in particular bronchodilators. The measurement of maximal inspiratory pressure may also be useful in patients with cancer (12).
Multidimensional Assessment
Although some researchers have described a good correlation between the abnormality of pulmonary function tests and the intensity of subjective dyspnea (35), others found this correlation to be extremely poor. In some cases, the authors suggested that the lack of correlation between objective and subjective findings might be due to underlying psychiatric disorders (11).
Figure 25.2 shows the different stages in the production of dyspnea. These are common to other symptoms, such as pain. Neither the production nor perception of dyspnea can be measured. Expression of the intensity of dyspnea can be influenced by a number of factors described in the figure and should not be interpreted as a direct representation of the intensity of production of dyspnea at the level of mechanoreceptors or chemoreceptors.
The complex nature of dyspnea in many patients with cancer means that assessment of dyspnea in isolation is inappropriate and may result in overtreatment or inappropriate treatment of a patient’s expression of dyspnea. Multidimensional assessment of dyspnea using tools that look at dyspnea in the context of other physical and psychologic symptoms allows for determination of the part played by other factors such as pain, anxiety, depression, and somatization in the expression of dyspnea. The Edmonton Symptom Assessment System is an example of a validated multidimensional assessment tool (29, 44). Identification of the various factors influencing the expression of dyspnea in a given patient allows for the implementation of a multidimensional therapeutic approach for that patient.
Treatment
Treatment of dyspnea should focus on a patient’s expression of dyspnea rather than his or her apparent level of dyspnea as judged by tachypnea and use of accessory muscles of respiration, or the level of oxygen in the blood. It is not unusual for patients to have marked tachypnea and not report difficulty in breathing or conversely to report severe dyspnea in the absence of tachypnea.
Treatment approaches include therapies aimed at modifying a specific pathophysiologic cause and therapies aimed to provide general symptom control.
Treatments of Specific Pathophysiologic Causes
In patients in whom a specific cause of dyspnea is suspected, appropriate treatment of the potential underlying cause should be initiated. Treatment modalities include radiation therapy, often accompanied by high-dose corticosteroids for superior vena cava syndrome, and chemotherapy for some patients with pleural effusions or carcinomatous lymphangitis. High-dose corticosteroids may also be useful for patients with the latter. Drainage of pleural or pericardial infusions can give rapid relief. Other procedures, such as pleurodesis or creation of a pericardial window, may be necessary when effusions are recurrent.
Reversible airway obstruction is a common cause of dyspnea in patients with cancer who have a history of heavy smoking (45). In these patients optimum treatment of airway obstruction using bronchodilators, corticosteroids, and, if necessary, antibiotics for infective exacerbation of symptoms should considered. In addition, infections including pneumonia are responsible for the deaths of almost half the patients who die of advanced cancer (46). Dyspnea associated with pneumonia can be effectively treated with appropriate antibiotics.
In addition to the aforementioned causes, patients may present with severe anemia, massive ascites, an acute exacerbation of chronic asthma, or acute panic attacks as part of a chronic panic disorder. The latter is characterized by hyperventilation. Occasionally, metabolic acidosis associated with acute renal failure or lactic acidosis can result in hyperventilation. All these diagnoses should be considered during assessment of a patient with dyspnea and advanced cancer because specific therapy may result in rapid symptom relief.
Symptomatic Treatment
Symptomatic management of dyspnea is based on three main elements: oxygen therapy, drug therapy, and general measures of support and counseling. Evidence for the role of these three therapeutic approaches are discussed in subsequent paragraphs. For the purpose of this review, a Medline search of the literature on dyspnea published between 1966 and 2000 was conducted. All studies relating to the symptomatic therapy of dyspnea were reviewed. Studies were classified according to their methodology in three levels of evidence (45).
Oxygen
Long-term oxygen therapy has beneficial effects on the outcome of patients with COPD (47, 48). However, the symptomatic effects of this therapy are less clear. Some studies suggest that oxygen has no symptomatic effects in cancer dyspnea (49, 50). In addition, the evidence for a beneficial effect of oxygen in patients with congestive heart failure is also controversial.
Table 25.1 summarizes studies that have addressed the use of oxygen for symptom relief in a number of conditions. Only studies with level 1 evidence (randomized controlled trials) are discussed in this chapter. In the case of COPD, most studies suggest that there is a significant symptomatic improvement at
rest and during exercise as a result of the administration of supplemental oxygen (54, 56, 57, 58). However, Liss and Grant (55) found that the administration of 0, 2, or 4 L per minute of oxygen to patients with COPD was not superior to administration of air on resting dyspnea. Oxygen has been found to improve functional capacity in patients with COPD (56, 57).
rest and during exercise as a result of the administration of supplemental oxygen (54, 56, 57, 58). However, Liss and Grant (55) found that the administration of 0, 2, or 4 L per minute of oxygen to patients with COPD was not superior to administration of air on resting dyspnea. Oxygen has been found to improve functional capacity in patients with COPD (56, 57).
Table 25.1 Symptomatic Effect of Oxygen Therapy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In a systematic review of contemporary management of COPD, domiciliary oxygen therapy is the only intervention that has been demonstrated to prolong survival, but only in patients with resting hypoxia (63). These authors also found a significant increase in breathlessness after nasal anesthesia with lidocaine. The authors suggested that the reduction in breathlessness caused by nasal oxygen is a placebo effect caused by wearing the nasal cannula and is unrelated to gas flow or the increase of arterial oxygen tension. In the case of congestive heart failure, one study found that supplemental oxygen could improve subjective scores for fatigue and breathlessness during steady-state exercise (61). Another study, however, found no significant benefit from supplemental oxygen on the symptomatic scores of patients subjected to regular walking (62).
Although the balance of evidence suggests that oxygen does have symptomatic effects in COPD and also probably in congestive heart failure, patients with dyspnea due to cancer most frequently have restrictive respiratory failure that might not respond to oxygen in the same way. There are three randomized controlled trials in which patients with cancer dyspnea were randomized in a crossover design to 5 L (51, 52) or 4 L per minute of oxygen (60). In the first two studies, all patients were hypoxemic on room air. In these patients, oxygen had a significant beneficial effect. In the third study, only 6 of 38 evaluable patients were hypoxemic; both oxygen and air were significantly superior to baseline (53). Swinburn et al. (54) studied the role of oxygen in a group of 10 patients with interstitial lung disease in addition to their main sample of 12 patients with COPD. Results in interstitial lung disease patients were as beneficial as those observed in the COPD group. It is possible that oxygen supplementation could improve function in patients with cancer dyspnea but not many studies have been conducted.
A Danish study aimed to evaluate the effect of noncontinuous oxygen therapy (NCOT) on pulmonary symptoms and sleep quality, and to determine whether patients with a subjective beneficial effect differed from those without effect in terms of patients’ characteristics, utilization of oxygen, hospitalization, and survival (64) 254 patients were prescribed oxygen <12 hour daily or to use as needed. Of these patients, 142 (55.9%) answered a questionnaire on hours spent with oxygen and symptomatic effect of oxygen treatment. While on oxygen, 76.3% of the patients reported improved dyspnea score (0–10) more than 0.5 points, 78.3% had improved quality of life, 59.5% improved sleep, 48.5% increased physical activity, 49.3% felt less tired and 40.0% reported improved thinking. Fifty-seven (43.2%) patients reported both improved dyspnea and physical activity whereas seven (5.3%) patients reported that oxygen had no effect on dyspnea but a beneficial effect on physical activity. Only 11 (7.7%) patients reported no subjective improvement on oxygen. Thus, NCOT improved symptoms and well-being in the majority of patients during daily activity, with most pronounced effects on dyspnea, sleep and quality of life. This effect was not significantly associated with number of hours spent with oxygen, underlying disease, hospitalization or survival. Very few patients sensed improved physical activity without relief in breathlessness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






