Factor
Literature (references)
Univariate
Multivariate
Patient (references)
Age
(17, 33)
(7, 13, 15, 16, 18, 22, 31)
Gender
NS
(18)
Tumour (references)
Histology
(7, 17)
(15, 31)
Site
(15, 23)
(7, 13, 17, 18, 22, 31, 33, 35)
Pattern
(13)
(17, 18)
Symptoms
NS
(22)
Timing
NS
(33)
Tg
NS
NS
Treatment (references)
RAI avidity
(13, 23)
(7, 15, 17, 18, 22, 33, 35)
RAI response
NS
(18)
Patient Factors
As is the case with primary treatment of localized DTC, age has proven to be a powerful prognostic indicator in patients with more extensive disease. In a series of 336 patients with distant metastases, Shoup et al. reported that an age of <45 years was associated with a 10-year disease-specific survival of 58 % compared with just 13 % in older patients [22]. Similar findings were published by Durante et al. in 2006 on a series of 444 patients [18]. The powerful impact of age is illustrated by smaller series in which it is often one of the only prognostic variables that consistently stands up to multivariate analysis [13, 16, 31]. In contrast to age, the evidence supporting the prognostic significance of gender is more variable. Whereas most series have failed to demonstrate significant outcome differences between men and women on multivariate analysis, Durante et al. reported a lower relative risk of death in women (RR = 0.7 [0.6–0.9]; p < 0.008) [18].
Tumour Factors
Numerous tumour factors have also been linked to prognosis. In particular, the site of metastatic disease appears to dictate outcome. As previously mentioned, the most common site of metastatic disease in DTC is the lung. Up to 75 % of patients will have pulmonary metastases and 40–55 % will have the lung as the only site of disease [6, 7, 15, 18, 22]. Nixon et al., in a review of 52 patients with metastatic DTC found that extrapulmonary metastases were associated with a lower overall 5–year survival (46 % vs. 75 %; p < 0.13) [31]. In addition, Durante et al. found that patients with lung-only disease were more likely to achieve negative imaging after RAI treatment, a pattern associated with 10-year survival in excess of 90 % [18]. This finding is somewhat surprising, given that the most commonly reported cause of death in thyroid cancer is respiratory failure secondary to replacement of lung tissue by cancer [26, 32]. Some authors have suggested that the pattern of lung disease may influence survival [17]. Micronodular, diffuse patterns (Fig. 6.1a, b) appear to be more favourable than macronodular disease (Fig. 6.2a, b), or associated mediastinal lymphadenopathy and/or effusion. Indeed, patients with micronodular disease were more common among those who achieved negative imaging after treatment in the aforementioned series by Durante et al. [18].
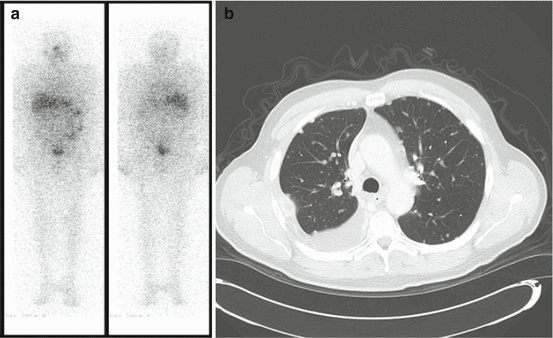
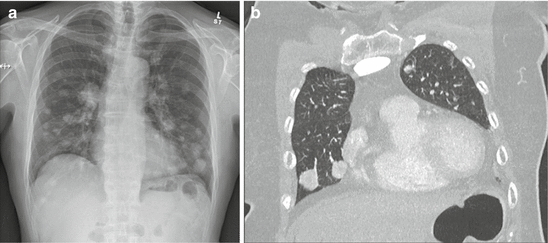

Fig. 6.1
(a) Radioactive iodine scan in a patient with micronodular lung disease. Increased diffuse uptake is seen throughout the lung fields. (b) CT scan in a patient with micronodular (<1 cm) lung metastases

Fig. 6.2
(a) Chest X-ray in a patient with macronodular lung metastases. (b) CT scan in a patient with macronodular lung metastases
Approximately 45 % of patients with metastatic DTC will have skeletal involvement [7, 13, 18, 22, 25, 33, 34]. Of these, bone is the only site in 25–40 %. The most common sites for bone metastases are the vertebrae, pelvis, ribs and femur. Published survival rates at 10 years vary from 15 to 38 % [7, 13, 18, 22, 25, 33–35]. Whereas this appears lower than survival rates published for pulmonary metastases, a number of large series have failed to establish significant differences in outcomes. In addition, bone disease is a direct cause of death in only a fraction of these patients [2, 25, 26, 32]. Therefore, it may be that bone disease simply reflects more advanced/resistant disease.
A smaller proportion of patients (12–20 %) will have multiple sites of disease [6, 18, 22]. Multiple sites of metastatic disease are not, surprisingly, associated with poor outcome. Shoup et al. reported a 17 % 10-year survival with two or more sites of metastatic disease compared with 32 % for lung-only and 27 % for bone-only metastases, a difference supported by multivariate analysis on 336 patients (p < 0.0001) [22].
Primary tumour histology might also be linked to prognosis in metastatic disease. Similar to the trend seen in overall survival for primary disease, patients with metastatic disease from FTC may fare more poorly than those with papillary subtypes. Sampson et al. reported a 3-year survival of 75 % for PTC metastases compared with 62 % for FTC (p < 0.006) [15]. However, larger series have found no such effect or findings that disappear on multivariate analysis. This observation may result from confounding factors, such as an increase in bone metastases in FTC as well as diminished avidity for and response to RAI [15, 18].
The presentation of metastatic DTC has also been examined for its potential significance. The following factors have been evaluated: (i) the timing of metastases (synchronous vs. metachronous), (ii) the presence of concomitant neck recurrence; (iii) the presence of clinical symptoms, etc. [6, 15, 16, 18, 22] None of these appear consistently to impact outcome in these patients. Finally, serum thyroglobulin (Tg) at diagnosis has been investigated, but any effect seems to disappear with multivariate analysis, suggesting this is simply a surrogate marker for other factors [15, 18].
Treatment Factors
Treatment-related factors have also proven to provide significant prognostic information. One of the most powerful variables studied appears to be the avidity of the disease for RAI and its subsequent response to therapy. Durante et al. identified three subsets of patients with respect to RAI avidity and response to treatment: (i) those with RAI-avid disease and negative imaging after treatment; (ii) RAI-avid disease but unable to achieve negative imaging with therapy; and (iii) no RAI avidity at diagnosis [18]. The 10-year survival rates for these groups were markedly different at 92 %, 29 % and 10 %, respectively [18]. Although still significant, not surprisingly, a number of confounding factors contributed to this large difference in prognosis. Specifically, patients in the most favourable group were more likely to be <40 years of age, have PTC and micronodular lung-only disease. Similarly, Casara et al. found RAI uptake to be an independent predictor of outcome in a review of 214 patients with metastatic DTC [7].
Summary
In summary, metastatic DTC has considerably worse overall outcomes compared to localized disease with survival estimated at ~40 % over 10 years. However, considerable variability exists within this group with respect to response to treatment and outcome. Young patients (<40 years) with lung-only, micronodular disease that is RAI-avid might have 10-year survival rates of >90 %. In contrast, older patients with multi-organ metastases and negative RAI scans have an expected long-term survival closer to 10 %. Clinicians involved in the care of these patients should consider these variables in advising prognosis and planning treatment strategies.
Diagnosis of Metastatic Disease
As previously noted, in 5–45 % of patients with distant disease, metastases are diagnosed at the time of initial presentation [1, 6, 15, 18, 19, 22, 25, 26]. In these patients the extent of disease becomes evident on preoperative cross-sectional imaging or post-therapeutic RAI scans. In a small percentage of patients, the metastatic disease itself is the initial presentation of DTC [9, 12, 15, 16, 19, 31, 36]. The remaining patients will have their metastatic disease discovered during routine follow-up.
Long-term follow-up in DTC is crucial, as recurrence may occur 10–20 years after initial treatment. Post-cancer surveillance recommendations rely heavily on neck ultrasound and serum Tg measurements [1]. Diagnostic RAI scans are used selectively in specific patients. The emphasis on neck imaging is supported by the observation that most recurrences are located in regional nodal basins [1, 3, 4]. However, a rising Tg with negative neck imaging suggests the possibility of distant metastatic disease.
An elevated serum Tg with no evidence of regional recurrence is appropriately investigated by diagnostic RAI scanning [1]. Unfortunately, 10–15 % of such patients will have a negative scan [1, 37–42]. This lack of sensitivity could result from insufficient dosing or problems in patient preparation with respect to thyroid hormone withdrawal or low iodine diet [37, 38]. Truly negative scans in this setting may be a consequence of tumour dedifferentiation with loss of cell membrane expression of the sodium-iodide symporter [12, 37, 38, 43–47].
Subsequent work up in these patients is somewhat controversial. Use of cross-sectional imaging and traditional radiographs in this setting is limited by poor sensitivity. Some authors have supported the routine administration of therapeutic-dose RAI [41, 42, 48]. Pacini et al. found evidence of uptake on 30/42 patients with elevated Tg and negative diagnostic scans following administration of therapeutic doses (90–150 mCi) [42]. Similarly, Pineda et al. demonstrated metastatic disease on post-therapy scans (150–300 mCi) in 16/17 patients [48]. Unfortunately, neither report could show any impact on outcomes. A recent review concluded that whereas it is not uncommon for uptake to be demonstrated with a therapeutic dose, evidence of clinical benefit is insufficient to recommend this practice [40].
Recently, the utility of FDG PET/SPECT in these patients has been examined [49–56]. Routine use of this expensive, limited resource is not justified, given the low risk of metastases and its limited sensitivity when applied broadly. Wang et al. performed both RAI and FDG SPECT in 239 patients being followed for DTC [54]. The overall sensitivities of both RAI scanning and FDG SPECT in patients with elevated Tg were just 48.7 % and 50.4 %, respectively. However, a ‘flip-flop’ phenomena was noted in which FDG SPECT was more likely to be positive in the setting of a negative RAI scan. In fact, the sensitivity of FDG SPECT was significantly higher in this setting (89.7 %) compared to when these traditional tests were positive (18.6 %). Similar findings have also been reported by a number of other authors [49, 57]. Schluter et al. demonstrated that the accuracy of FDG PET appears to be correlated with increasing serum Tg levels [53]. In this series, true positive rates were 11 %, 50 % and 93 % in the setting of Tg levels of <10, 10–20 and >100 ug/L, respectively. Given this, the authors suggested that this test is more appropriately targeted to those patients with a serum Tg of >10 ug/L.
The use of FDG PET/SPECT may also provide prognostic information and guide treatment. Unfortunately, non-iodine avid, FDG PET/SPECT positive disease appears to be resistant to traditional treatment with 131I. Wang et al. reported a series of 25 patients with FDG-avid disease, all of whom received at least one dose of 131I [56]. At follow up the median volume of disease, SUVs and serum Tg had all increased in comparison to a non-randomized control group. Thus, information provided by this diagnostic test may aid in guiding therapy or selecting patients for clinical trials.
Current American Thyroid Association (ATA) guidelines support the consideration of FDG PET/SPECT in patients with elevated Tg and negative RAI scans (grade C) [1]. In addition, these guidelines acknowledge potential emerging roles in a number of other areas that include the following: (i) as a prognostic tool in distant metastatic disease; (ii) selecting patients unlikely to respond to traditional RAI; and (iii) measuring outcomes of other local or systemic therapies.
The role of cross-sectional imaging is less clear and typically guided by symptoms. [1] Chest CT scans or plain radiographs may reveal anatomical evidence of lung metastases. Cross-sectional imaging may be used to confirm rare brain metastases when suggested by symptoms or extensive disease at other sites. Also, traditional 99mTc bone scintigraphy has a sensitivity approaching 80 % for the detection of bone metastases [50]. However, in the setting of FDG PET/SPECT it is probably redundant, given that PET has a similar sensitivity (85 %) and superior specificity (99 % vs. 91 %) for the detection of bone metastases in these patients [50].
In summary, in 5–45 % of patients that develop distant disease, metastases are evident at the time of diagnosis on cross-sectional imaging or post-therapy RAI scans. The remainder will have distant disease detected during follow-up. In patients with an elevated serum Tg and no evidence of regional disease a search for distant metastases may include diagnostic whole-body RAI scanning. Those patients with elevated Tg and negative scans present a clinical dilemma. More recently, FDG PET/SPECT has shown promise as a diagnostic tool in these patients. The role of FDG PET/SPECT in metastatic thyroid cancer will continue to evolve as more data become available.
Management of Distant Metastases
Given the broad range of outcomes in distant metastatic disease, management of these patients requires multidisciplinary care. Treatment plans may need to be tailored on a case-by-case basis after consideration of factors related to the patient, tumour and subsequently modified according to the response to treatment. Given the relative rarity of metastatic disease in DTC, guidance from large randomized control trials is lacking. As such, management guidelines must derive recommendations from smaller trials, cohort studies and case series. Interestingly, despite the considerable heterogeneity in this patient population, the initial mainstays of treatment are similar and include the following: (i) TSH (thyroid-stimulating hormone or thyrotrophin) suppression; (ii) RAI; and (iii) surgery [1].
TSH Suppression
TSH stimulates thyroid cell proliferation and thus has long been hypothesized to play a potential role in thyroid cancer recurrence. Consequently, TSH suppression with levothyroxine in doses above those required for ‘replacement’ has been proposed in the management of thyroid cancer. While some conflicting evidence exists, larger studies and meta-analyses have been able to demontrate a link between TSH and a reduction in adverse outcomes [1]. In a study of 2036 patients registered in a multi-institutional thyroid cancer registry, Jonklaas et al. demonstrated improved overall survival in patients with stage II, III or IV disease [4]. Interestingly, whereas a dose-response relationship with the level of TSH suppression was observed in those with more advanced disease (stages III and IV), suppression of the TSH beyond the initial subnormal range in stage II disease did not produce a further incremental benefit. Currently, the ATA recommends aggressive suppression of TSH (<0.1 mU/L) in people with persistent disease but a more conservative approach in those with high-risk (0.1–0.5 mU/L) and low-risk (0.3–2 mU/L) disease in order to prevent recurrence [1]. Clearly, the benefit derived from TSH suppression must be balanced against the potential detrimental effects on cardiac and bone health in each patient.
Radioactive Iodine
RAI ablation following surgery is frequently employed in the primary treatment of DTC. Potential benefits include: (i) facilitating cancer surveillance with serum Tg by ablation of remnant thyroid tissue; (ii) potential reduction in risk of recurrence/disease-specific mortality; and (iii) identification and treatment of persistent disease [1]. When persistent/recurrent metastatic disease is identified, RAI is usually the preferred initial treatment as long as the disease remains iodine-avid [1, 7, 12, 18, 22, 30, 31, 33, 34, 58–61]. The published data on this topic, whereas largely retrospective, does suggest that RAI is of utility in guiding prognosis and might confer a survival benefit.
In addition to iodine avidity, a number of other factors appear predictive of the response to RAI. Durante et al. published a series of 444 patients with distant metastatic disease from PTC or FTC [18]. Of these, 295 were found to have iodine-avid metastatic disease. All patients were treated empirically with 100 mCi. In the setting of persistent iodine-avid disease, treatments were then repeated at 3–12-month intervals until negative studies were obtained. No fixed limit on maximum cumulative dose was set. Overall, 127 patients achieved negative imaging studies during follow-up. A large proportion of these occurred later in the course of therapy (>5 years). In this subset of patients, 10-year survival was 92 % compared to 29 % in those with iodine-avid disease and persistently positive imaging. The 10-year survival of patients without 131I at the outset was just 10 %. The authors examined the characteristics of patients in the most favourable group and found that they were more likely to be young, (<40 years) with PTC, and pulmonary site-only metastases with no findings or micronodular disease on cross-sectional imaging [18]. A number of additional studies have published similar findings supporting the benefit of RAI [6, 7, 10, 15, 22, 34, 58, 61].
Although not universal’ most studies seem to indicate that pulmonary disease responds more favourably to RAI than metastases at other sites [7, 15, 18, 22, 36, 61]. Most studies show that the rate of RAI avidity (~67 %) is similar between lung and bone metastases. However’ bone metastases appear to be more advanced at the time of diagnosis as most are visible on plain radiographs or cross-sectional imaging [18, 36]. Thus’ it would be more reasonable to expect outcomes comparable to those seen in macronodular pulmonary disease. In these subsets’ evidence continues to suggest a survival benefit associated with RAI treatment but complete responses are rare [10, 13, 17, 18, 22, 23, 33, 35, 59–61]. As a result’ additional therapies play a larger role in the treatment of bone disease.
Current recommendations strongly support the use of RAI in patients with micronodular, iodine-avid disease. In these patients repeated doses should be given at 6–12 month intervals while the disease continues to concentrate iodine and until negative results are achieved [1]. In addition macronodular pulmonary and bone disease may be treated as long as objective benefits are observed [1]. A single safe dose limit has not been defined and the decision to terminate RAI treatment should be individualized to the patient.
The use of therapeutic dose RAI in patients with non-avid disease on diagnostic scans has been an area of considerable controversy. A number of studies have been able to demonstrate an increased sensitivity of post-therapeutic (>100 mCi) whole-body scans compared to diagnostic (<6 mCi) studies [37, 40–42, 48]. Pacini et al. demonstrated uptake on 30/42 post-therapeutic scans in this clinical setting [42]. Those 30 patients were given additional RAI treatments. A mean of three treatments per patient were administered (cumulative range of doses 90–500 mCi) over a period of 6.7 years. Unfortunately’ when compared to an untreated group of 28 similar patients’ no significant difference in Tg levels or clinical outcomes were observed. More recently’ a systematic review published by Ma et al. concluded that insufficient evidence existed to support this approach [40]. Consistent with this assertion’ the updated ATA guidelines do not recommend the use of RAI in these groups [1].
The positive outcomes associated with RAI in appropriate patients and the lack of efficacy seen with other treatments for metastatic disease have led to interest in agents that might enhance iodine uptake and retention in non-avid tumours [12, 43–47, 62, 63] Impaired iodine (131I) uptake appears to be related to diminished cell membrane expression of the sodium-iodide symporter. Retinoic acid’ via its binding to the retinoic acid receptor’ regulates transcription of genes related to cell differentiation. In vitro studies demonstrate some extent of redifferentiation with these compounds [64, 65]. As a result, cells increase their expression of the symporter and take up iodine more efficiently. Unfortunately, clinical studies have failed to show clinical benefit consistently. Courbon et al. reported a series of 11 patients with metastatic DTC and no uptake on whole-body scans [43]. All were treated with 13-cis-retinoic acid. RAI uptake, a surrogate clinical marker of redifferentiation, increased marginally in merely 2 patients. After a mean follow-up of 24 months, 5 patients had died, 5 showed evidence of progression and only one had stable disease. Zhang et al. used another isomer—all-trans-retinoic acid—in 11 patients with advanced-stage DTC and poor RAI uptake [47]. Increase in RAI uptake was seen in 4 patients and diminished uptake in 6. Partial responses were observed in 5 patients and progressive disease was noted in 4 of them. Currently, the use of these compounds outside of a clinical trial cannot be recommended [1].
Lithium has been shown to prolong the duration of RAI uptake by inhibiting its release from the cell [62]. Unfortunately, this effect is not mediated through increase in sodium-iodide symporter expression and thus is only of potential benefit in tumours that are already iodine-avid. Rosiglitazone, a thiazolinedione drug used in patients with diabetes, has shown some early promise in thyroid cancer redifferentiation [44–46]. Rosiglitazone is a peroxisome proliferator-activated receptor gamma agonist. This transcription factor is a regulator of cellular differentiation and has been shown to inhibit tumour development in animal models and human tissue cell lines. Phase II studies have demonstrated some efficacy in increasing RAI uptake in patients with metastastic DTC and negative whole-body scans [44]. Kebebew et al. reported the clinical results of rosiglitazone in 20 patients with DTC and negative RAI whole-body scans [45]. Five patients demonstrated an increase in RAI uptake after treatment. A reduction in Tg level post-therapy was seen in 3 patients. None had a partial response by RECIST criteria and 7 had progressive disease. Thus, whereas this drug shows promise with respect to tumour redifferentiation and increased RAI uptake, significant clinical benefit has yet to be demonstrated. Finally, gene therapy with the introduction of the sodium-iodide symporter gene into tumour cells using an adenovirus vector has been investigated, but its efficacy seems to be limited by short duration of action [63, 66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree