Introduction
The unrelenting increase in the global prevalence in diabetes mellitus has meant that the burden of its associated complications is expected to increase significantly over the next few decades. Diabetic foot disease (DFD) is perhaps the most feared of all such complications leading to a high degree of individual and societal burden . The rate of lower extremity amputation (LEA) continues to rise in many countries —for example, in the United Kingdom, 169 of such procedures are performed every week. The mortality risk is substantial, with the 5-year mortality from DFD being worse than many cancers . The economic budern is also enormous—the estimated direct costs of diabetic foot care in England was equivalent to £1 in every £140 spent by the National Health Service in 2014–15 . Incident new DFD could add between $11,710 and $16,833 incremental burden to an individual’s annual healthcare expenditure in the United States, doubling the cost of delivering diabetes care . There are considerable invisible indirect costs which are more difficult to estimate such as loss of earnings effects of employee absenteeism and the impact on careers and family. The reported rate of diabetes-related foot complications in low- and middle-income countries is higher, occurring at a relatively younger age .
Nonetheless, many recent improvements and innovations have led to a significant improvement in the outlook for the individual with diabetic foot. In this chapter we discuss the multidisciplinary management of the DFD, focusing on diabetic foot ulceration (DFU) and Charcot neuroarthropathy (CN). We explore the contemporary principles of care including the evolving role of surgical specialties within the diabetic foot.
Management of diabetic foot ulceration
DFU has a lifetime incidence between 19% and 35% in individuals with diabetes. It is defined as skin breakdown of the foot (at the level or below of the malleoli) penetrating the dermal layer as a minimum. In the western hemisphere, the annual incidence of DFU is estimated to be around 2%, although rates as high as 8% in other geographical locations have been reported . Prevalence rates of DFU are between 5% and 13% depending on the populations and health systems evaluated. The triad of loss of protective sensation (LOPS), ischemia, and foot deformities come together in DFU, and the clinical course is often complicated by infection. Therefore, the key principles of DFU care are centered around prompt recognition of ulceration, timely recognition of ischemia and the provision of high-quality standard care (SC) (to include wound care, timely treatment of infection, revascularization where needed and the provision of quality offloading) preferably in a multidisciplinary setting. Equally, concurrent optimization of the many medical comorbidities that individuals with DFU experience is essential .
Multidisciplinary diabetic foot service
Modern DFU care is provided through a collective of healthcare professionals working in collaboration for the benefit of the patient—termed a “multidisciplinary” diabetic foot service (MDfS). This allows for a systematic, consistent, and coordinated delivery of care, often under a single auspice. Significant improvement in DFU healing rates and reduction in major amputation rates have been universally reported with the development of such services . In addition, improved mortality rates (by up to 20%) and reduction in the duration of hospitalization (up to 40%) and medical treatment costs have been reported . The components of the MDfS team varies globally but as a minimum includes podiatry, vascular surgery, physicians with diabetes experience and a orthotist but larger units will have orthopedics, plastics, infectious diseases, specialist musculoskeletal radiologists, and physiotherapy teams providing close support. It is notable that many countries presently do not offer training programs in podiatry with the role being often handled by physicians or nursing personnel with wound care expertise in many countries.
Management from ulceration to healing
Responsiveness of healthcare structure: time to first expert assessment
Timing of referral is crucial. Longer time to first expert assessment is associated with worse DFU prognosis . DFU that have experienced a delay in referral are often graded as severe during their first expert review . The challenge to ensure individuals with new DFU are prioritized and referred early is a global one, including for health systems that are deemed advanced. In one recent exploratory study across Europe, 48% of patients were referred longer than 1 month after first reporting then to their primary care team . During the COVID-19 pandemic which strained healthcare resources worldwide, suboptimal DFU outcomes alongside a delay in first expert review have been noted, underscoring the importance of ensuring a robust pathway . Patient factors may also contribute to a delay in expert assessment—those with LOPS may neglect the presence of DFU and infection as they do not experience the pain and discomfort that would typically raise alarm. Guidance and toolkits to improve time to referral are available , some with adaptations to cope with the inexorable service reorganization that has been mandated by the COVID-19 pandemic .
Classification of diabetic foot ulceration
Ulcer classification and grading systems facilitate the communication of ulcer severity and serial progression between various members of the MDfS, homogenize DFUs recruited into studies and audits, but importantly, influence treatment strategies . Numerous classifications and scoring systems with varying degrees of validation exist . The University of Texas classification (UTC) and the Wagner classification systems are widely used in clinical practice and research. Site, Ischemia, Neuropathy, Bacterial Infection, Area and Depth (SINBAD) is utilized in the United Kingdom National Diabetes Foot Audit, which by virtue of its simplicity, allows for evaluation of high-volume data between multiple institutions . The Wound, Infection, Ischemia (WIfI) facilitates the assessment of ischemia and the likely benefit of revascularization and has been shown to predict risk of LEA and DFU healing in cohorts ; but its use to prognosticate outcomes of individual DFU is not recommended.
Key assessments at first visit
At first assessment, a detailed history to understand the context of the DFU is needed. This should include estimating the duration of ulceration. In addition, a detailed medical, drug and personal history should be elicited. Examination of the foot should include assessments for LOPS and foot perfusion. Ulcer characteristics should then be determined including DFU site, size, depth (superficial or probe to the bone) and infection status and graded using an ulcer classification system. Blood tests are recommended to assess overall health, estimate level of systemic infection (if ulcer appears infected) and current glycemic control. Imaging choices will be guided by the clinical picture. Table 15.1 details the various blood, vascular, imaging, and microbiological assessments that should be considered at the first patient visit to an MDfS. Comment should also be made of current footwear, ambulatory status, and if any mobility aids are used by the individual.
| Tests at first DFU assessment in an MDfS | |
|---|---|
| Hematology | Full blood count, erythrocyte sedimentation rate (ESR) |
| Biochemistry | Urea and electrolytes, liver functions, CRP, HbA1C, lipid profile |
| Radiology |
|
| Vascular lab |
|
| Microbiology |
|
Standard care in the management of diabetic foot ulceration
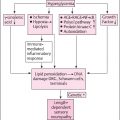
SC in DFU is the algorithmic and protocolized provision of the best possible treatment supported by an evidence-based and cost-effectiveness data, individualized to the patients’ needs, to achieve DFU healing/remission. The key SC spokes include wound control through regular debridement and the judicious use of dressings, identification of ischemia and revascularization where needed, infection control, offloading, and metabolic control. Ensuring pain relief and continued surveillance after healing to maintain remission are also increasingly considered part of SC ( Fig. 15.1 ).
Wound control: debridement and dressings
The TIME framework (tissue debridement, inflammation control, moisture balance, and epithelialization of wound edges) underscores the key elements of wound control . Regular debridement of devitalized and necrotic tissue promotes wound healing by activating repair and reducing the bacterial load. Scalpel or “sharp” debridement is considered as the gold standard, as it triggers the wound healing process by swiftly changing the surface of a chronic wound into an acute wound but provides the added advantage of relieving pressure created by callus ( Fig. 15.2 ). It can be undertaken in the clinic, or if extensive ulceration is present, may also be performed in a surgical theater facility. It is very unusual for patients with DFU to require an anesthetic for sharp debridement. Limitations of the technique include that it can on occasions be painful, requires careful practice and not all practitioners are allowed to perform them.

Other available methods include mechanical, enzymatic, or autolytic debridement techniques. Examples of mechanical debridement include hydrosurgical, using pressurized irrigation, deep cleansing with moistened gauze, brushing with monofilament pads, and the use of ultrasound as a tissue disruptor . In enzymatic debridement, gradual chemical liquefaction of the devitalized tissues is observed after topical application of agent. Water-soluble proteinases such as collagenase and papain are typical examples. The use of larvae-based debridement (maggot debridement) has been popular for many decades , being particularly helpful when there is significant necrotic tissue that is too painful for sharp or mechanical debridement. Autolytic debridement using hydrogels is a very slow process best suited for dry necrotic wounds in care facilities where limited expertise is available to offer other forms of debridement and the individuals themselves are too frail to visit a hospital clinic. The recent consensus statement from the International Working Group on the Diabetic Foot (IWGDF) noted that while there is clear consensus supporting debridement for wound cleansing, the superiority of a single technique over another could not be established . It is therefore important to consider factors such as pain, operator confidence, need for swift control of necrosis, and costs of debridement when considering the optimal approach. However, in the presence of acute severe infection, sharp surgical debridement is the mainstay.
The application of the dressing to a DFU is intended to relieve symptoms, protect the wound and support healing . A wide array of dressings are now available with some modern dressings capable of targeting specific aspects of healing. The wound bed in DFU is notoriously dynamic with a progressive temporal impact from infection, undulations in peri-wound and leg edema, impact of patient mobilization and adherence to the offloading provided. Therefore, it is unlikely that a single dressing type or formulation can support the ulcer over the entirety of its clinical course. Nonadherent dressings such as saline-soaked gauze are simple to use, inexpensive, and for many years, have been used as SC in control arms of dressing trials. Highly exuding wound require dressings that support moisture control such alginate or foam dressings (with additional hydrocolloid or hydrofiber layers) which are absorbent and effective for heavily exuding wounds . Hydrogel-based dressings can moisturize dry, eschar heavy wound and consequently facilitate autolytic debridement but their use in dry gangrene needs to be carefully monitored for signs of progression to wet gangrene at the interface of dead and healthy tissue. Dressings containing iodine and silver are regarded for their antiseptic potential, used typically when local infection is present. All dressings should be changed frequently, and occlusive dressings ideally avoided in infected DFUs. However, presently, there is no evidence to support the use of one dressing type over another as SC in DFU, a fact that has been reiterated in the most recent consensus statements from the IWGDF and the American Podiatric Medical Association/Society for Vascular Medicine . The only exception to this is the recommendation to consider sucrose-octasulfate potassium salt impregnated dressing as an adjunct to SC in difficult-to-heal neuroischemic DFU without infection .
Ischemia and vascular control
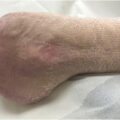
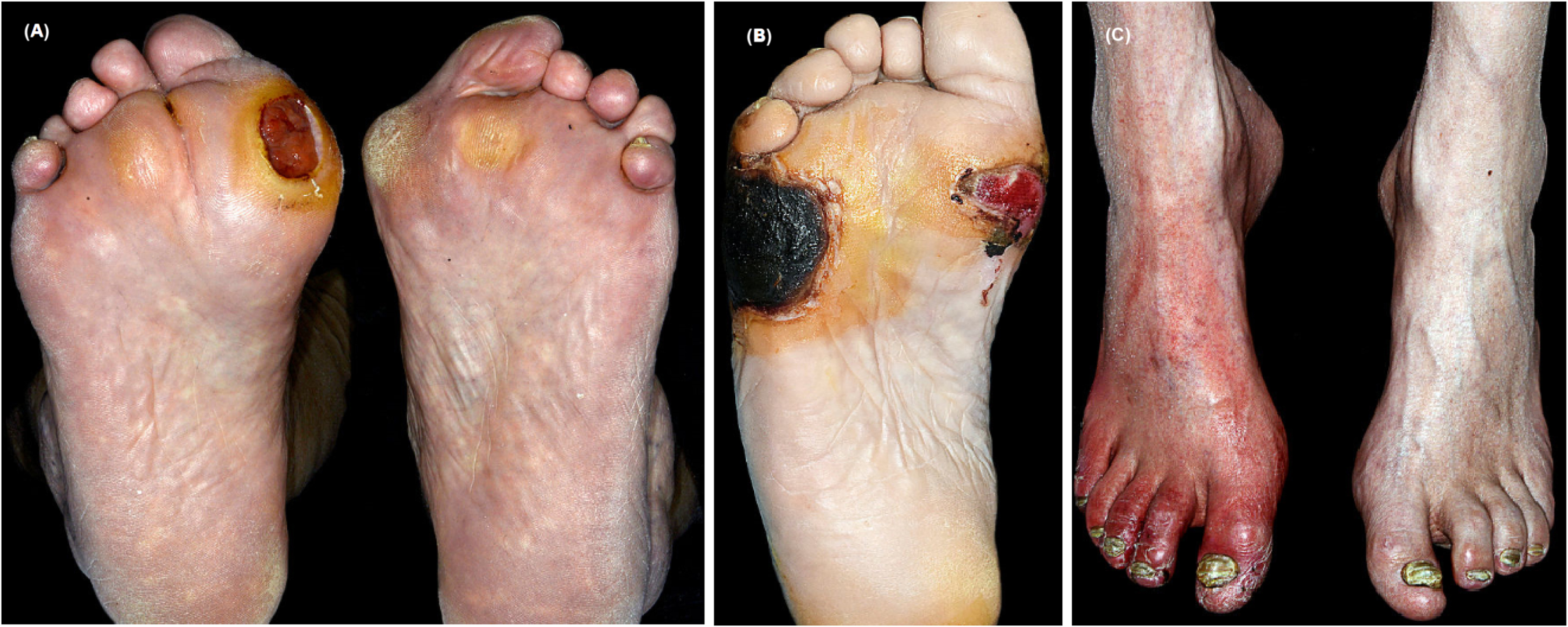
It is understood that ischemia complicates 45%–60% of all DFU with prevalence rates becoming evident over the last few decades. It is a clinical spectrum ranging from subtle peripheral arterial disease (PAD) which may have negligible impact on wound healing to more established chronic limb threatening ischemia (CLTI). In itself, CLTI represents a clinical continuum of increasing degrees of ischemia which can delay wound healing and increase risk of amputation . Early characterization of limb perfusion advisable, if possible, on the first visit itself as the outcome will help guide the DFU management options. Neuroischemic DFUs are usually seen on the nonweight-bearing sites of the foot such as the medial and lateral aspects of the feet or the tips of the toes. These regions are often in contact with footwear and experience shear forces during mobilization but may also occur spontaneously . The callus formation surrounding the ulcers is limited, thin, and shiny. This distinguishes neuroischemic DFU from classical neuropathic DFU which typically occur on weight-bearing plantar surfaces overlying bony prominences and often display thick callus overgrowth. The loss of protective sensation also means that individuals with diabetes often do not report intermittent claudication but rather present directly with tissue loss. Thus a common presentation of CLTI in diabetes is a foot with features of critical limb ischemia and areas dry gangrene indicating regions of direct cutaneous infarction ( Fig. 15.3 ). Individuals with diabetes, with or without ulceration, can also develop acute limb ischemia (from emboli, acute trauma, graft thrombosis, or nonatherosclerotic conditions) which has a distinct clinical phenotype and needs immediate support from the vascular surgical specialists. Importantly, individuals undergoing dialysis have higher prevalence of neuroischemia resulting in lower wound healing and greater amputation rates compared to those not receiving dialysis .
The decision to revascularize an individual with DFU and ischemia will depend on multiple factors such as severity of perfusion abnormalities identified, size of ulceration, extent of tissue loss, and infection with restoration of pulsatile in-line flow to the affected region being the primary goal. Those with milder perfusion abnormalities can be observed closely within the MDfS with SC provision and a decision to revascularization made if the DFU deteriorates or when progress is unpredictably slow. Recent advances in interventional radiology alongside ultradistal/pedal bypass surgery within an integrated pathway have facilitated delivery of complex revascularization .
Offloading
The primary aim of adequate offloading is to reduce abnormal pressure over the ulcer and redistribute it to the area surrounding the DFU to promote healing. A wide array of offloading choices is available, with varying degrees of clinical effectiveness to support their use such as the total contact cast (TCC), removable prefabricated cast walkers, custom splints and extra-depth shoes, surgical boots, and half-shoes. In addition, the use of wheelchairs, crutches, and scooters can also be recommended to support with achieving the balance between desired offloading and maintaining quality of life. Among these, the nonremovable TCC is recommended as the first choice for neuropathic forefoot or midfoot plantar ulcerations . However, in those with neuroischemic DFU, TCC is a relative contraindication. If a TCC is not tolerated or contraindicated, options include a removable knee-high or ankle-high boot. Padding the peri-ulcer skin with semicompressed adhesive felt combined with proper fitting footwear may be adequate in small DFUs. Those with chronic or recurrent ulcerations resulting from high-pressure points related to bony deformities should be considered for surgical offloading. Advocating complete nonweight bearing to individuals with DFU is impractical and is not recommended, except in specific situations (e.g., postmajor bony debridement or postorthopedic reconstruction) and even then, for a limited period only. Ensuring adherence to offloading and recommendations on mobilization remains a challenge.
Infection control including osteomyelitis
It is estimated that half of all DFUs become infected during their lifetime. Diabetic foot infection (DFI) can lead to delayed healing, greater odds of hospitalization . The severity of infection can range from mild with features localized around the ulcer, moderate with more locally extensive features including extension to subdermal structures including tendon or bone, and severe infection presenting with systemic inflammatory response alongside limb-threatening features . Imaging with plain radiography and magnetic resosnace imaging (MRI) should be undertaken to asess the extent of the infection and confirm the presence of osteomyelitis. Microbiological specimens are critical to identify the pathogen—tissue sampling is encouraged where such expertise exists; ideally bone specimens are encouraged for the confirmation of ostemyelitis. In those with mild infection, debridement of surrounding callus, slough and necrotic tissue may be sufficient, contributing to reducing the microbial bioburden. The selection of an antibiotic agent will depend on the likely pathogen (or proven by culture) as well as allergies, drug interactions, and the cost. In most European countries gram-positive organisms such as Staphylococcus aureus and Streptococcus spp. are the most commonly encountered pathogens . However, polymicrobial isolates, including a wide array of gram-negative organisms such as Klebsiella spp., Escherichia coli , and Pseudomonas spp. and anaerobes are common in more chronic DFU . In Africa and Asian regions, gram-negative organisms may be presenting pathogen, sometimes declaring multidrug resistance at the onset .
For soft tissue infection ( Fig. 15.4 ), empirical antibiotic therapy should target gram-positive organisms ( as a minimum) , however, DFUs that are chronic or isolating antibiotic-resistant organisms may require bespoke antibiotic treatment plans form the outset. Co-amoxiclav 625 mg thrice daily is our empiric antibiotic of choice for mild infections in individuals without penicillin allergy. The high prevalence of antibiotic-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), and carbapenem-resistant organisms is emerging as a major challenge in determining antimicrobial therapy . The recent IWGDF consensus statement has recommended a treatment duration of 1 or 2 weeks for mild soft tissue infection, with longer doses to be considered in the presence of slow healing or PAD . Antibiotic treatment should be combined with other principles of SC including debridement.
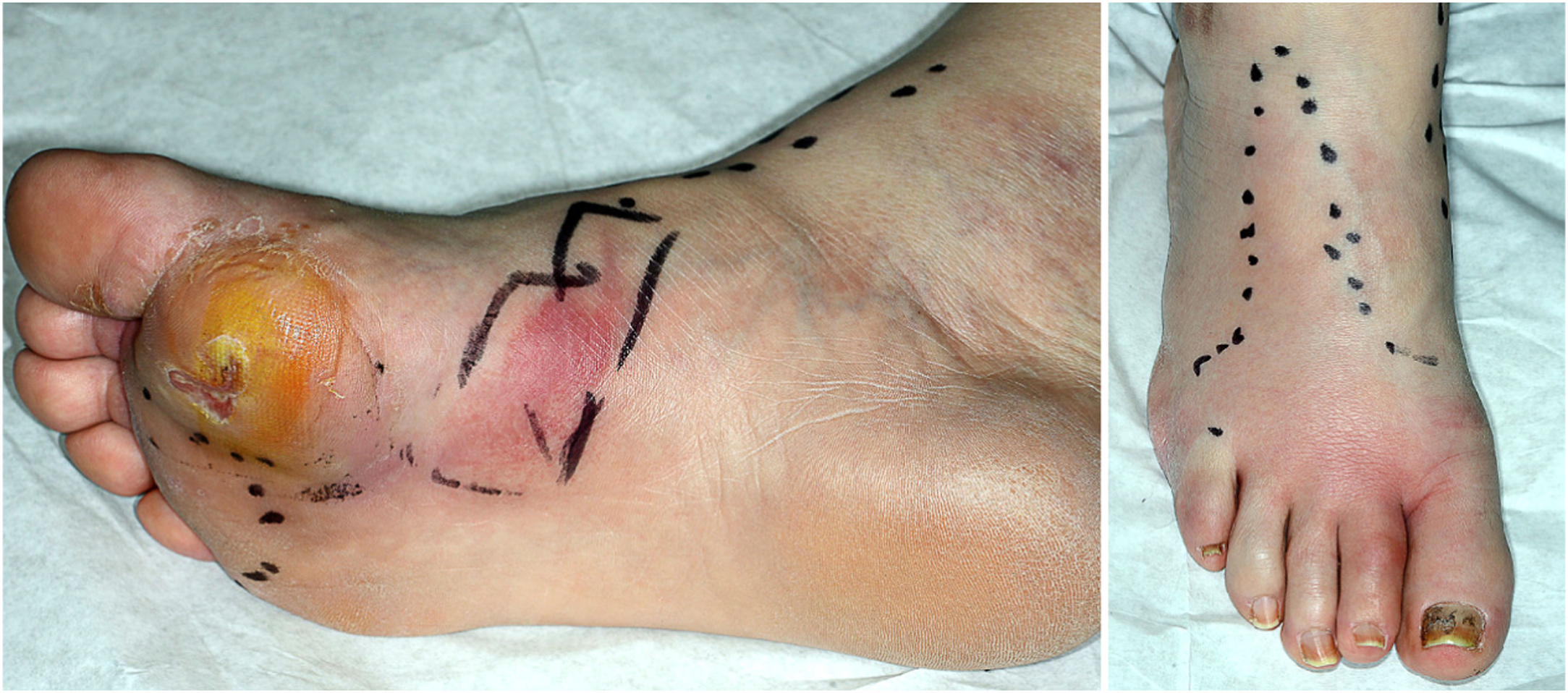
Infections exhibiting moderate or severe features are considered as potentially limb threatening. These may require hospitalization and possibly surgical debridement. Indications for urgent surgical intervention, among others, include rapidly spreading necrosis, deep abscess with systemic features and gas in the soft tissues on X-ray examination. A consensus on the best choice of antimicrobial agent or combination is lacking but in the presence of severe soft tissue infection (with or without DFO) broad-spectrum intravenous antibiotics such as Co-amoxiclav 1.2 g thrice daily or tazobactam-piperacillin 4.5 g thrice daily along with a glycopeptide such as teicoplanin or vancomycin (in those with suspected or known MRSA infection), are the empirical agents of choice—the glycopeptide is typically discontinued once absence of MRSA is confirmed. Targeted antibiotic regimes are guided by the microbiology results.
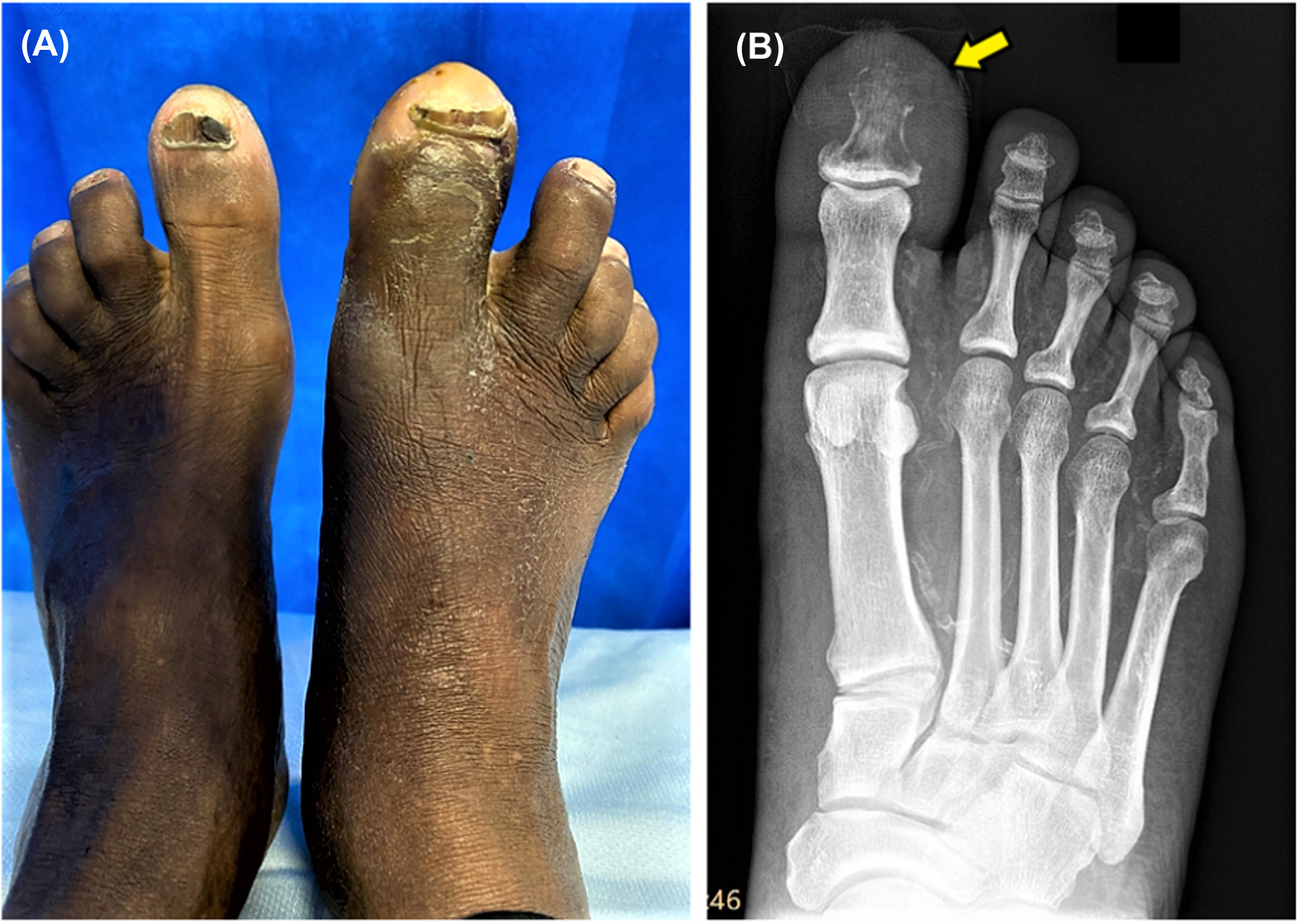
Diabetic foot osteomyelitis (DFO) is common, occurring in nearly one in five of moderately infected and one in two of severely infected DFU . Severe DFO (acute or chronic) typically displays active soft tissue infective component, however chronic DFO can exist with only mild extrinsic soft tissue features. Presence of a swollen toe or foot, history of long-standing ulceration or exacerbations of foot infection every time antibiotics are stopped should raise concerns of DFO. Findings include presence of a deep ulcer (or a sinus) which probes to bone. Plain radiography can reveal evidence of cortical destruction or fragmentation ( Fig. 15.5 ) but has poor sensitivity, especially in early DFO and over bony resection areas. In comparison, MRI has a high sensitivity (>90%) and specificity (>90%) for the radiological detection of DFO and can also provide insight to the extent of soft tissue involvement .
Management of DFO consists of bone debridement along with removal of any sequestrum, targeted antibiotic therapy guided by bone specimen culture and ongoing intensive DFU care. Antibiotic therapy should be offered for 6 weeks but if there is no clinical improvement within 4 weeks, further inquiry into slow progress and antimicrobial confirmation is required . The recently published OVIVA study, while not specifically designed to evaluate response in DFO, has demonstrated the safety and clinical effectiveness of early switch from intravenous to oral antibiotics with no differences in outcomes between the early oral switch group and the group receiving only intravenous therapy for the study duration . There is evidence to support the notion that primary antibiotic therapy only may be adequate, but this is mainly in forefoot predominant, stable (low-grade) osteomyelitis without evidence of necrotizing infection, managed through an MDfS with close podiatric support . There is limited consensus on the optimal mode of antibiotic delivery and recent evidence suggests that short courses of antibiotics may suffice if a high-quality source control has been achieved . Surgical bone debridement should be considered, as part of SC, in DFO with recurrent soft tissue infections, extensive midfoot and hindfoot osteomyelitis or when infection control is not achieved as predicted or there is a strong suspicion of nonviable infected bone .
Diabetic foot attack
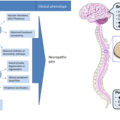
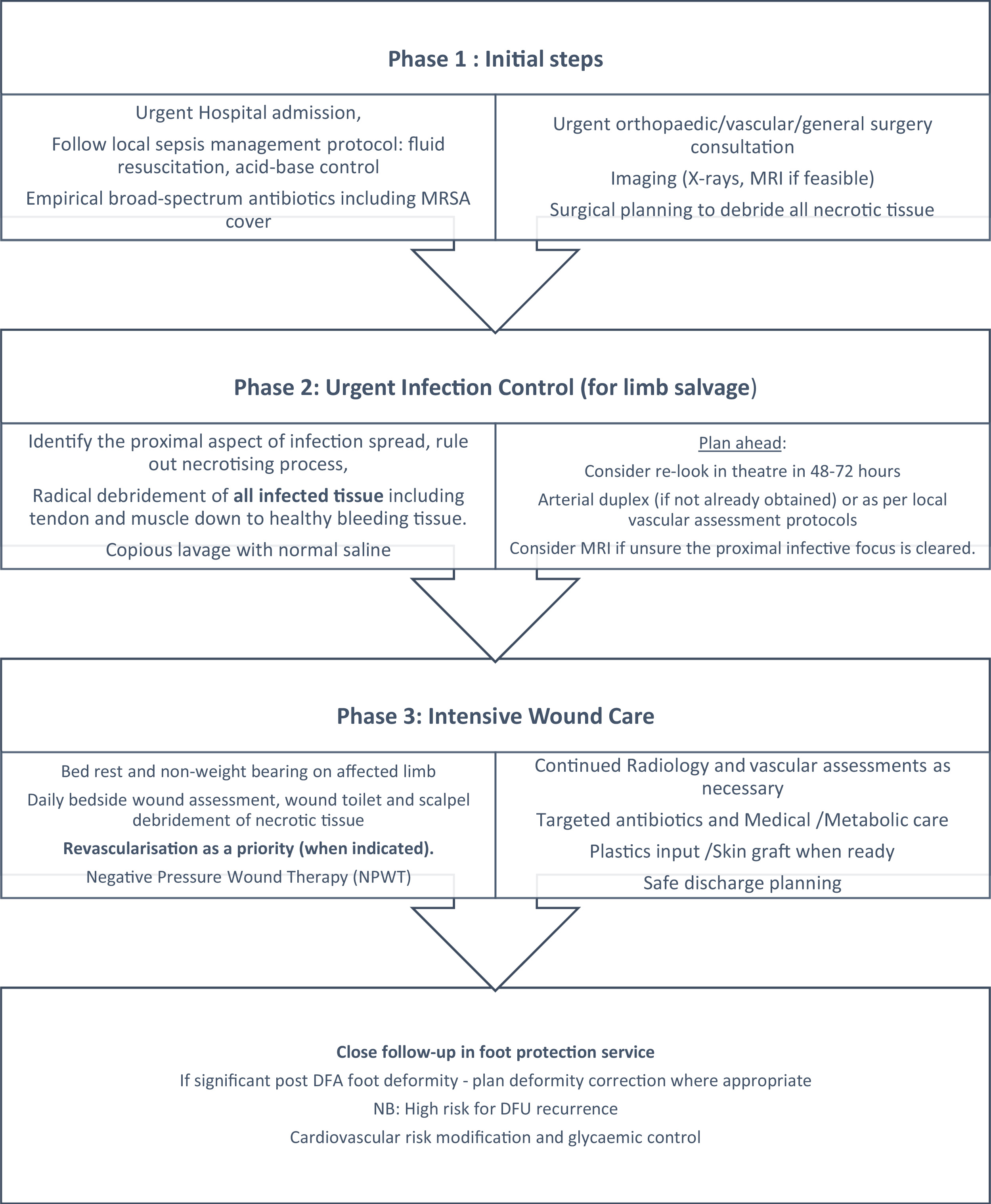
Not infrequently, DFUs can present with rapidly spreading infection and ascending cellulitis, tissue necrosis, and systemic inflammatory syndrome. Such a presentation can potentially escalate into a limb threatening scenario without timely intervention and is labeled as a “Diabetic Foot Attack” . Without urgent (at times, immediate) intervention, it carries a significant risk of a major amputation and threat to life. Management of a diabetic foot attack (DFA) frequently involves admission to hospital, initial broad-spectrum empirical antibiotics, medical stabilization, and urgent surgical debridement of all infected tissue followed by culture-guided antibiotic therapy, high-quality wound care and where necessary, revascularization . Soft tissue defects occurring following radical debridement can be managed with negative pressure would therapy (NPWT) and early skin grafting when granulation has reached the surface of the wound. Fig. 15.6 describes a phased approach to the management of a DFA, which is best undertaken in a multidisciplinary setting. Such a protocolized approach has demonstrated high rates of healing and limb salvage .
Evaluating response to standard care treatment
The percentage area reduction (PAR) in DFU can be considered a useful guide of DFU progress during SC provision. Individuals with >50% reduction in PAR by 4 weeks are up to six times more likely to progress toward healing by 12–20 weeks . However, the evidence is based around classically neuropathic DFU. It may not transfer easily to the prediction of healing in neuroischemic DFU and therefore requires cautious interpretation. Measurement of biomarkers such as levels of wound fluid matrix metalloproteinases (MMPs) or tissue inhibitors of MMP or inflammatory cytokines such as interleukin-6 (IL-6) remain very much research tools and are presently not geared toward application into frontline practice .
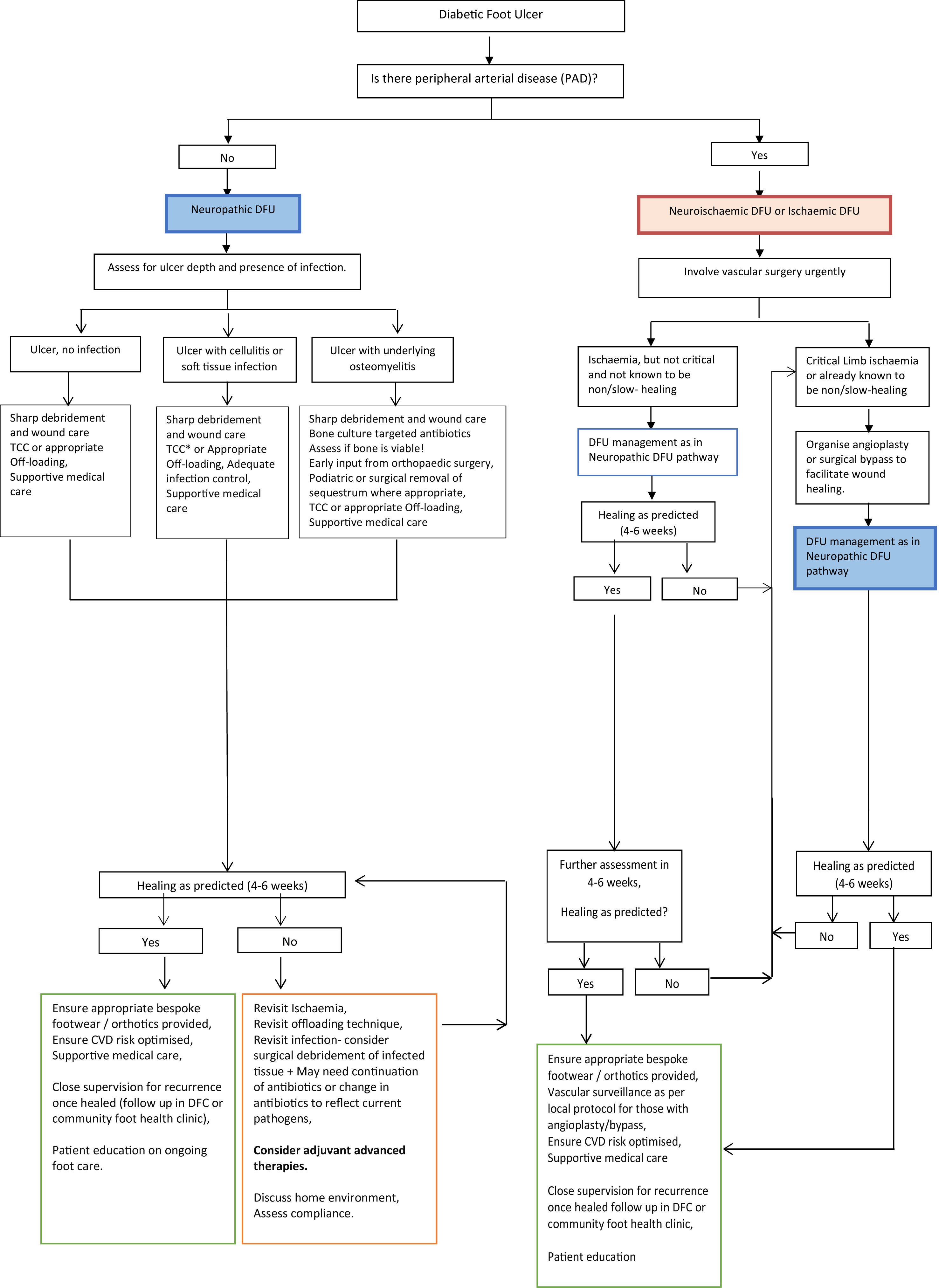
The King’s College Hospital SC approach to healing a DFU is described in Flowchart 15.1 .
Advanced therapies in diabetic foot ulceration
Despite the wide-ranging components of SC, the healing of foot ulcers remains a challenge. In the United Kingdom National Diabetic Foot Audit, approximately half of new DFUs healed within the first 12 weeks and among those still alive at 12 months, a quarter were removed unhealed . Provision of adjuvant advanced wound healing therapies may therefore be attractive in difficult-to-heal DFUs or when wound progress has simply stalled . The effectiveness of these interventions can be variable, and their role is supplementary to SC. Indeed, it is important to ensure that any significant ischemia is corrected beforehand (or at times, alongside) and adequate offloading and infection control interventions are in place. A few of the important interventions include negative pressure wound therapy (NPWT), hyperbaric oxygen therapy (HBOT), artificial skin substitutes, growth factors including platelets and their derived products, acellular dermal matrices and physical therapies such as laser and electromagnetic applications . While a detailed overview is out of scope of this chapter, a brief description is provided.
Negative pressure wound therapy
NPWT is used to enhance the healing of acute, chronic (converted to acute through debridement) or closed incisional wounds. The device applies negative pressure to the ulcer/wound bed thereby providing an airtight seal using an electrically powered vacuum pump. This leads to enhancement in local blood flow, removal of excess exudate and stimulation of granulation. A Cochrane meta-analysis reported that NPWT increased proportion of healed wounds compared with moist wound therapy, a faster healing time and reduction in amputation risk . The evidence for NPWT in DFU healing is stronger in postsurgical diabetic foot wounds than for chronic ulcers offered NPWT after receiving clinic-based debridement or wound cleansing only . The standard pressure of NPWT application is 125 mmHg; evidence for the efficacy and safety of lower suction pressures is not available.
Oxygen therapies
Oxygen is the essential element in wound healing as it modulates many biological processes that progress the wound from the proliferative stage to the remodeling stage of healing. Chronic hypoxia can lead to inadequate collagen synthesis, downregulates angiogenesis and reduces antimicrobial defense, contributing to formation of a chronic, static wound .
Adjunctive treatment with HBOT was the first available technology. It consists of individuals either alone or in groups breathing 100% oxygen at 2.2–2.5 atmospheric pressure (atm) of oxygen in pressurized chambers daily for several weeks. Early studies indicated HBOT benefitted amputation avoidance . The Hyperbaric Oxygen Therapy in Diabetics with Chronic Foot Ulcers (HODFU) trial reported higher healing rates of ischemic and neuroischemic DFU with HBOT compared to placebo over a 12-month period . The two recent studies did not demonstrate a benefit with HBOT but had significant methodological limitations and/or subjective endpoints . One challenge in interpreting results of the many trials of this technology is the significant variation in study methodologies and heterogeneity of clinical end points used. A recent meta-analysis of 11 studies concluded that adjuvant HBOT lowered the major amputation rate but not improve DFU healing. Individuals with significant frailty and cardiac failure are unable to tolerate HBOT . Furthermore, access of the technology is limited in most countries and its cost-effectiveness in DFU remains unproven. The IWGDF has recommended consideration of HBOT as an adjunct in nonhealing ischemic or neuroischemic DFU already receiving best standard of care but the strength of recommendation was low as it was based on moderate quality evidence .
Topical oxygen therapy (TOT) involves the process of direct wound oxygenation with either continuous delivery or intermittent (cyclical) pressurized systems. The equipment used is portable or can be easily installed at home. One study reported nearly twofold higher rate of complete DFU closure at 12 weeks with continuously diffused topical oxygen therapy . More recently, a study using cyclical pressurized topical wound oxygen (TWO 2 ) technology reported a four times increased likelihood of DFU healing at 12 weeks compared to SC alone . A topical spray containing purified hemoglobin, which can bind oxygen from the local atmosphere and subsequently diffuse it into the wound bed has shown early promise . While the technology can be arguably viewed as in its infancy, the results from the studies are encouraging. The future adjunctive use of TOT may appeal to both individuals with difficult-to-heal DFU and healthcare providers.
Placental membrane-derived products
Placental membranes contain components necessary for stimulating wound healing such as collagen-rich matrices, growth factors, cytokines in addition to viable endogenous cells such as mesenchymal stem cells (MSCs), neonatal fibroblasts, and epithelial cells which induce angiogenesis and potentiate wound repair . Formulations available include cryopreserved membranes (containing live cells) and dehydrated chorionic amniotic membrane products where live components are removed by a purification process. The advantage with dehydrated products is that they do not require refrigeration and have a longer shelf life. After debridement and wound cleansing, the product is applied directly on top of the ulcer base and covered with nonpermeable dressing. In their 2019 update, the IWGDF concluded that placental-derived products may lead to a higher wound closure rate in difficult-to-heal DFU when compared to standard of care only . The technology is expensive and cost-effectiveness data is limited .
Growth factors
Stalling of tissue regeneration in the inflammatory phase can lead to chronic wounds. A lack of growth factors has been implicated in chronic wounds with several trials have explored the clinical effectiveness of products that contain one or more of such growth factors for stimulating wound healing. Commercially applications are now available to use as adjuvant wound therapy contain factors such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), or a combination of FGF and EGF . The use recombinant platelet-derived growth factor (r-PDGF, Becaplermin) is approved by the Food and Drug Agency (FDA) for the treatment of difficult-to-heal DFU without ischemia or infection and not penetrating into deeper structures of the foot . However, Becaplermin is expensive and efficacy data has been inconsistent between studies and therefore has not been widely recommended in international guidelines . A Cochrane review which examined trials of 11 different growth factor formulations reported a positive signal, albeit weak, to support superior healing of DFU compared to SC only but no effect on amputations . Another recent innovation is a patch dressing of autologous leukocytes, platelets, and fibrin (Leucopatch 3C Patch, Reapplix Inc.) applied directly on the DFU demonstrating a higher healing rate compared to SC in difficult-to-heal DFU including those with mild neuroischemia .
Bioengineered skin and acellular dermal products
A number of skin substitutes are available. They can be either cellular, containing either allogeneic or autologous cells, or acellular matrices comprised of collagen subtypes and chondritin . They provide an extracellular matrix scaffold to support fibroblast migration and angiogenesis. Studies have reported apparent increased rate of DFU healing when compared to SC only, but the studies have generally been at moderate to high risk of bias . The exact role of these products in the healing algorithm is still being debated and current guidance does not fully recommend their use .
Physical therapies
Several physical therapies have claimed efficacy in the healing of DFU. Evidence of apparent superiority in DFU healing is available for devices using laser , ultrasound (mainly used a tool for debridement to stimulate granulation) , therapeutic magnetic resonance , electrical stimulation , and phototherapy/radiation, but in general, the quality of evidence supporting their use is low . Their use is currently not recommended in any consensus documents on DFU healing.
Metabolic control and treatment of associated comorbidities
A brief overview is provided in Table 15.2 . The risk of diabetic micro- and macrovascular complications is greater in those with suboptimal glucose control which in turn increases the risk of developing DFD. Lower HbA1C (<7% or 53 mmol/mol) has been shown to lower the risk of amputation but the impact of short-term improvement in glycated hemoglobin (HbA1C) on DFU healing has been less obvious . However glycemic control should be encouraged given the pleiotropic benefits of improved glucose control—from reducing the potential for DFU infection, around perioperative/periprocedural outcomes and cardiovascular health .