Management of Advanced Hepatic Disease
Daniel Y. Chung
David A. Sass
Cirrhosis is a pathologically defined entity that is associated with a spectrum of characteristic clinical manifestations. Histologically, it represents a late stage of progressive hepatic fibrosis characterized by distortion of the hepatic architecture and the formation of regenerative nodules. This stage of advanced liver disease is generally deemed “irreversible” and the only therapeutic option may be liver transplantation. When patients with cirrhosis experience “decompensation,” they are susceptible to a variety of complications and, as a result, they have a markedly reduced life expectancy. Cirrhosis was the tenth leading cause of death in the United States, according to a 2000 Vital Statistics Report, in which data was collected through 1998 and accounted for more than 25,000 deaths (1).
This chapter focuses on the medical management of patients with cirrhosis. Most of the chapter will deal with the specific management of cirrhotic-related complications. Mention will also be made of the indications for and evaluation of such patients for liver transplantation.
When treating patients with cirrhosis, it is important to first confirm the diagnosis. Thereafter, the physician should attempt to establish the etiology of the disease, assess severity, identify and treat any complications, and decide whether an evaluation for an orthotopic liver transplant (OLT) is indicated. Finally, family counseling regarding screening for genetic liver diseases, when appropriate, and administration of vaccines should also be offered.
Confirming the Diagnosis of Cirrhosis
Patients are diagnosed with cirrhosis in one of several ways:
They may have stigmata of chronic liver disease discovered on routine physical examination (Table 29.1)
They may undergo routine laboratory testing, radiologic testing [ultrasound, computerized tomography (CT) scan or magnetic resonance imaging], endoscopy (with the discovery of varices indicating portal hypertension), or an unrelated surgical procedure that incidentally leads to the diagnosis of cirrhosis
They may present with one of the life-threatening complications of decompensated cirrhosis ab initio
They may undergo a liver biopsy for further evaluation of abnormal liver enzymes and be found to have hepatic cirrhosis histologically
They may never come to clinical attention and be diagnosed at autopsy (up to 30–40% in older reviews) (2).
Table 29.1 Stigmata of Chronic Liver Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The gold standard for diagnosing cirrhosis is examining the explanted liver at autopsy or following liver transplantation where the architecture of the entire liver can be appreciated. In clinical practice, however, we usually rely on examination of a pathologic specimen obtained through transjugular or percutaneous liver biopsy. There have been numerous radiologic advances over the past several years, particularly with regard to CT scanning. These improvements include changes in scanner technology such as the ability to perform multiphasic contrast-enhanced studies as well as better intravenous contrast media. Nevertheless, none of the radiologic modalities has yet supplanted liver histology as the gold standard. However, liver biopsy is not always necessary to confirm a diagnosis of cirrhosis if the clinical, laboratory, and radiologic data strongly suggest the presence of cirrhosis, for example, a patient with ascites, severe coagulopathy, and a shrunken, nodular liver on ultrasound. In this instance, a liver biopsy may be hazardous as the coagulopathy may pose a significant bleeding risk.
Determining the Cause of Cirrhosis
Determination of the cause of cirrhosis is important because it may influence treatment decisions and counseling of family members and may help predict complications [e.g., the development of hepatocellular carcinoma (HCC) is higher in patients with hemochromatosis and chronic hepatitis B than in other forms of cirrhosis]. A specific etiology can often be determined by way of history combined with serologic and histologic evaluation. The two most common causes in the United States are alcoholic liver disease and hepatitis C, together accounting for approximately half of those undergoing transplantation. When no apparent cause of cirrhosis is identified, the term “cryptogenic” is used (approximately 10–15% of cases). Many of the patients who were previously labeled as “cryptogenic” are now recognized as being cases of unrecognized nonalcoholic steatohepatitis (NASH). For a full classification of the many etiologies of cirrhosis and the best diagnostic tests, see Table 29.2.
Table 29.2 Causes of Cirrhosis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Assessing the Severity of the Disease
Several prognostic tools have been developed to assess the severity of disease in patients with cirrhosis and the need
for OLT. The Child-Turcotte-Pugh (CTP) classification, which was initially designed to stratify the risk of portocaval shunt surgery in patients with cirrhosis and variceal bleeding, has gained favor over the past decade as a simple method for determining the prognosis of patients with chronic liver disease (3) (see Table 29.3 for scoring system and survival rates). In 1997, the United Network for Organ Sharing (UNOS) established a CTP score of 7 or higher as the minimal listing criteria for eligibility of listing for OLT (4). On February 27, 2002, UNOS adopted the model for end-stage liver disease (MELD) score as an evidence-based means of organ allocation (5). MELD score is a severity score predictive of mortality in patients with chronic liver disease (6). It includes total serum bilirubin, international normalized ratio (INR) and serum creatinine:
for OLT. The Child-Turcotte-Pugh (CTP) classification, which was initially designed to stratify the risk of portocaval shunt surgery in patients with cirrhosis and variceal bleeding, has gained favor over the past decade as a simple method for determining the prognosis of patients with chronic liver disease (3) (see Table 29.3 for scoring system and survival rates). In 1997, the United Network for Organ Sharing (UNOS) established a CTP score of 7 or higher as the minimal listing criteria for eligibility of listing for OLT (4). On February 27, 2002, UNOS adopted the model for end-stage liver disease (MELD) score as an evidence-based means of organ allocation (5). MELD score is a severity score predictive of mortality in patients with chronic liver disease (6). It includes total serum bilirubin, international normalized ratio (INR) and serum creatinine:
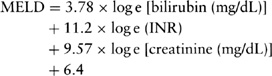
Table 29.3 Child-Turcotte-Pugh Score to Assess Severity of Liver Disease | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
MELD was initially developed to predict the survival of patients undergoing transjugular intrahepatic portosystemic shunts (TIPS) (7) and was subsequently validated in patients with decompensated liver disease (6). Using the MELD model, patients are assigned a score on a continuous scale from 6 to 40,
which equates to estimated 3-month survival rates from 90 to 7%, respectively (7). The MELD score has been shown to be useful in predicting short-term survival in groups of patients on the waiting list for OLT (8) as well as the risk of postoperative mortality (9). Once patients are listed for OLT, an updated MELD score is recalculated at varying intervals as a form of status recertification, according to the following schedule: every 12 months for those with a MELD ≤10; every 3 months for those with a MELD of 11–18; every month for those with a MELD of 19–24, and every week for those with a MELD of 25 or greater (http://www.optn.org/PoliciesandBylaws2/policies/docs/policy_8.doc).
which equates to estimated 3-month survival rates from 90 to 7%, respectively (7). The MELD score has been shown to be useful in predicting short-term survival in groups of patients on the waiting list for OLT (8) as well as the risk of postoperative mortality (9). Once patients are listed for OLT, an updated MELD score is recalculated at varying intervals as a form of status recertification, according to the following schedule: every 12 months for those with a MELD ≤10; every 3 months for those with a MELD of 11–18; every month for those with a MELD of 19–24, and every week for those with a MELD of 25 or greater (http://www.optn.org/PoliciesandBylaws2/policies/docs/policy_8.doc).
The Transplant Evaluation Process
As soon as it has been determined that a patient is sick enough to require consideration for an OLT and that no other alternative treatments are available, a careful evaluation should be performed to assess the following:
Whether the patient would survive the operation and the immediate postoperative period
Whether the patient can be expected to comply with the complex medical and immunosuppressive regimen required post-transplantation
Whether the patient has any other comorbidities that would severely compromise graft or patient survival because of which transplantation would be futile and an inappropriate use of a scarce donor organ (10).
In assessing whether any given patient with cirrhosis is a suitable candidate for OLT, the evaluation process typically involves a multidisciplinary team including transplant hepatologists, transplant surgeons, transplant anesthesiologists, nurse coordinators, social workers, and transplant psychiatrists with expertise in substance abuse issues. Other consultants will also be called upon according to individual patient needs. The evaluation includes a thorough history and physical examination, a full psychosocial assessment, extensive laboratory testing, and abdominal imaging to exclude intra- as well as extrahepatic malignancies and to evaluate biliary and hepatic vascular anatomy. An extensive cardiopulmonary evaluation as well as age-appropriate screening is performed for various extrahepatic malignancies. On the basis of the pre-OLT evaluation testing, a multidisciplinary selection committee makes a decision regarding the candidacy for OLT listing. The natural history of disease should be compared with the expected survival after OLT. Current survival rates 1, 3, and 5 years after liver transplantation in the United States are 88, 80 and 75%, respectively (http://www.optn.org/latestdata/step2.asp).
Management of Specific Complications
Ascites
Ascites, or the pathologic accumulation of fluid in the peritoneal cavity, is the most common major complication of cirrhosis. It is associated with a poor quality of life, increased risk of infection and renal failure, and a poor long-term outcome (11). Approximately 50% of patients with compensated cirrhosis will develop ascites over a 10-year period. Furthermore, only 50% of patients with cirrhosis survive 2–5 years after ascites onset, depending on the cause of liver disease (12). Cirrhosis accounts for 80% of all cases of ascites. Other etiologies are listed in Table 29.4. Defining the etiology of ascites and confirming that it is secondary to end-stage liver disease is critical, as successful treatment is dependent upon an accurate diagnosis.
Table 29.4 Causes of Ascites (and Their Frequencies) | |||||||
|---|---|---|---|---|---|---|---|
|
The pathophysiology of ascites in liver disease involves splanchnic vasodilatation. In advanced stages of disease, arterial blood volume decreases and arterial pressure falls largely because of circulating vasodilators such as nitric oxide. There is a compensatory response of vasoconstrictors and anti-natriuretic factors leading to sodium and fluid retention. Intestinal capillary pressure and permeability are altered and the result is accumulation of abdominal fluid (11).
Patients with ascites frequently seek medical attention within a few weeks of onset. They often become intolerant of the abdominal distension leading to early satiety and shortness of breath. In the initial evaluation of patients with ascites, a careful history documenting risk factors for liver disease should be taken. Evidence of malignancy, cardiac disease, myxedema, renal disease, pancreatitis, and infections such as tuberculosis should all be ruled out as etiologies of ascites (12). In addition, more than one of these may be found in 5% of patients with ascites (13). Abdominal distension occurs if a moderate amount of ascites is present. Diagnosis can be made by percussing the flanks. Flank dullness can be detected if >1500 mL of fluid is present. If no flank dullness is present, the patient has a < 10% chance of having ascites (14). A useful test for diagnosing ascites is examination for shifting dullness. The flank is percussed in the supine position and the air-fluid level marked. The patients are then turned on their side and percussion is again performed. If the air-fluid level shifts upward, it indicates that ascites is present. Other maneuvers such as the puddle sign and fluid wave have been described but their utility is often questioned (15). Many recommend some form of imaging in patients with ascites to not only confirm the presence of ascites but also to evaluate for cirrhosis and/or malignancy. Ultrasound of the abdomen is usually sufficient and can detect as little as 100 mL of fluid in the peritoneal cavity (12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






