Fig. 1
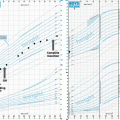
Weight development in childhood-onset adamantinomatgous craniopharyngioma patients recruited in HIT Endo according to hypothalamic involvement. Body mass index (BMI) SDS is shown at time of diagnosis and at two intervals after diagnosis (8–12 years and more than 12 years). White boxes: BMI at diagnosis; hatched: 8–12 years follow-up; black: more than 12 years follow-up. The horizontal line in the middle of the box depicts the median. The top and bottom edges of the box, respectively, mark the 25th and 75th percentiles. Whiskers indicate the range of values that fall within 1.5 box-lengths. Reproduced from Sterkenburg et al. [11] with kind permission of Oxford University Press
Epidemiology
Craniopharyngioma is rare, with an incidence of 0.5–2 cases per million persons per year, 30–50% of all cases presenting during childhood and adolescence [16, 17]. Craniopharyngioma represents 1.2–4% of all childhood intracranial tumours. In the pediatric age group, its histological type is usually adamantinomatous with cyst formation [18–21]. Craniopharyngioma can be detected at any age, even in the prenatal and neonatal periods [18]. A bimodal age distribution has been shown, with peak incidence rates in children of ages 5–14 years and adults of ages 50–74 years. No gender differences have been observed in population-based studies. Craniopharyngioma cases have been reported within two families but an underlying genetic susceptibility has not been verified [22, 23]. Metastases are rare events in childhood-onset adamantinomatous craniopharyngioma, mainly occurring in the corridor of surgical approach [24].
The German Pediatric Cancer Registry (GPCR) systematically documents cases of childhood-onset adamantinomatous craniopharyngioma [25]. The GPCR data from 1980 to 2007 obtained for 496 childhood-onset craniopharyngioma patients diagnosed ≤18 years reveal most patients (451; 91%) were younger than 15 years of age at initial diagnosis, with a 1:1 sex ratio and a median age at initial diagnosis of 8.8 years. The 1980–2007 overall survival rate was 97% after 3 years from diagnosis, 96% after 5 years, and 93% after 10 years. Patients diagnosed with childhood-onset craniopharyngioma in the 1980s had a lower overall survival rate than patients diagnosed in the 1990s (overall survival at 5 years, 91% vs. 98%) [25, 26].
Pathogenesis and Molecular Pathology
Craniopharyngiomas are non-glial intracranial tumours derived from a malformation of embryonic tissue [27]. There are two differing hypotheses on its embryonic origin: originating from residual embryonal epithelium of the anterior infundibulum and the anterior pituitary gland versus originating from ectodermal remnants of Rathke’s pouch [27].
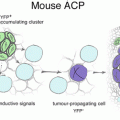
Recent observations have given insight into biological markers of pituitary tumours [28]. Beta-catenin is highly expressed in adamantinomatous craniopharyngioma, whereas in papillary craniopharyngioma beta-catenin expression is absent [29]. Beta-catenin has been identified as a downstream component of the Wnt signal pathway that regulates cellular proliferation, development, and morphology [28]. Mouse studies have shown that only pituitary embryonic precursors or adult stem cells are able to generate tumours when targeted with oncogenic β-catenin , which suggests that the cell context is critical in order for mutant β-catenin to exert its oncogenic effect. Interestingly, mutant stem cells do not generate the bulk of the tumour cells; instead, they induce tumours in a paracrine manner. Combining basic studies in mice and humans will provide further insights into the biology of these neoplasms and will reveal pathogenic pathways that could be targeted with specific inhibitors for the benefit of patients [30].
Presenting Clinical Manifestations
The diagnosis of childhood-onset craniopharyngioma is often made late—sometimes years after initial appearance of symptoms [25, 31]—with a clinical picture often dominated by manifestations of intracranial pressure (e.g. headache and nausea) [32, 33]. Further primary manifestations are endocrine deficits (52–87%) and visual impairment (62–84%). Hormonal deficits are frequently caused by disturbances to the hypothalamic–pituitary axes that affect growth hormone secretion (75%), gonadotropins (40%), thyroid-stimulating hormone (TSH) (25%), and adrenocorticotropic hormone (ACTH) (25%). At the time of initial diagnosis, 40–87% of patients present with at least one hormonal deficit [34], and other endocrine symptoms such as central diabetes insipidus are present preoperatively in 17–27% of all patients [34, 35]. An analysis of anthropometric data obtained in routine checkups before the diagnosis of childhood-onset adamantinomatous craniopharyngioma in 90 patients [36] revealed that a pathologically reduced growth velocity—an early manifestation of the disease—presents in patients as young as 12 months, but that significant weight gain, predictive of hypothalamic obesity, tends to occur as a later manifestation, shortly before diagnosis.
Hoffmann et al. [37] concluded from their analysis of clinical presentation at diagnosis and duration of history in 411 patients with childhood-onset adamantinomatous craniopharyngioma that childhood-onset craniopharyngioma is frequently diagnosed after very long duration of history, especially in older children and adolescents. However, long duration of history before initial diagnosis was not a risk factor for large tumour size or impaired outcome in terms of survival or functional capacity. Visual impairment and neurological deficits in history should be considered as symptoms necessitating rapid diagnostic work-up. Growth failure and weight gain are early symptoms in history, which should lead to early consideration of craniopharyngioma in differential diagnosis.
Risk-Adapted Treatment Strategies
Novel risk-adapted treatment strategies are focusing on the following main goals: (a) reversal of visual compression symptoms, (b) relief of raised intracranial pressure, (c) prevention of tumour regrowth/progression, and (d) restoration or substitution of pituitary hormone deficits plus all other supplement-supportive measures, while minimising acute and long-term mortality and morbidity [2–4].
Surgery
De Vile et al. [38, 39] published the first reports on the association between attempts at radical gross-total resection in case of hypothalamic involvement and long-term morbidity. Elowe-Gruau et al. [40] published an algorithm for surgical treatment of childhood-onset adamantinomatous craniopharyngioma patients, which recommends a hypothalamus-sparing strategy based on a grading of hypothalamic tumour involvement in preoperative magnetic resonance imaging (MRI) [41]. The authors reported that patients neurosurgically treated according to this algorithm using a hypothalamus-sparing approach had similar relapse rates and a lower prevalence of severe obesity than patients treated by gross-total resection (28% versus 54%, respectively). This was the first report in the literature proving the tolerability and efficacy of a hypothalamus-sparing strategy by comparing cohorts treated by the same experienced surgical team at a single institution, and thus eliminating the bias of surgical experience on outcome analysis. However, it is important to note that although the “hypothalamus-sparing surgery” increased the percentage of “normal” body mass index (BMI) from 17% to 38%, the likelihood of clinically significant weight gain remained 62% with nearly half of all patients developing morbid obesity. Based on this grading system, Garré et al. [27] published a modified algorithm for risk-adapted treatment strategies in childhood-onset craniopharyngioma. The authors emphasised the treatment by experienced neurosurgical teams and suggested proton beam therapy , especially for young patients (< 5 years of age) after limited hypothalamus-sparing surgery. Müller et al. [42, 43] published studies on a risk-adapted treatment strategy based on pre- and post-surgical grading of hypothalamic involvement/damage in MRI. The assessment of the suprasellar tumour extension towards the mammillary bodies is considered essential for their grading into anterior or posterior hypothalamic involvement/lesion (Fig. 2). According to their report, patients with post-surgical lesions affecting posterior hypothalamic structures presented with an increased BMI and reduced self-assessed quality of survival during long-term prospective follow-up. Mallucci et al. [44] published a treatment algorithm, suggesting a two-staged surgical approach with initial relief of cystic pressure and thereby down-staging the risk grade in appropriate cases. Van Gompel et al. [45] developed a MRI-based grading system to predict the risk of postoperative obesity in adults treated for craniopharyngioma. Irregular contrast enhancement and hypothalamic T2 signal change correlated and predicted more severe hypothalamic involvement. Larger postoperative weight gains correlated with progressive hypothalamic involvement. Steno et al. [46] reported an increased hypothalamic complication rate, in case of tumours with intraventricular extension as compared with purely extraventricularly located tumours. To preserve the hypothalamic function, the authors recommended to distinguish between extraventricular and intra- and extraventricular located suprasellar craniopharyngiomas. In the first group, the hypothalamus can be simply compressed and not infiltrated. Elliott et al. [47] used a newly designed Craniopharyngioma Clinical Status Scale (CCSS) to show that preoperative CCSS scores predicted postoperative outcome better than clinical characteristics like gender, age, location or tumour size, and the presence of hydrocephalus.
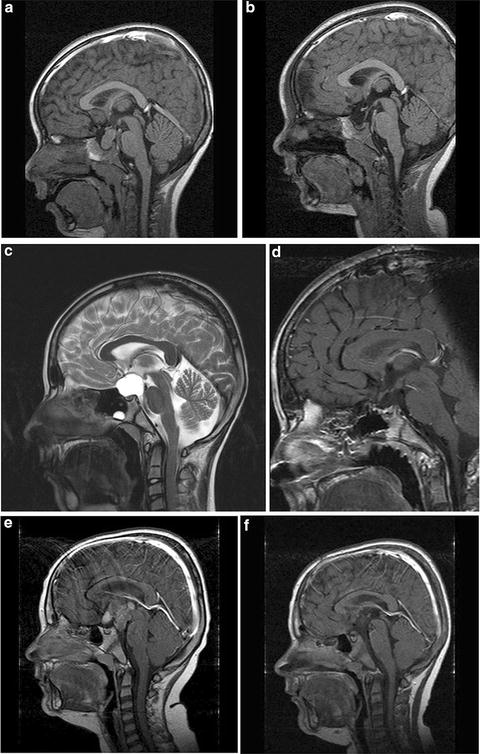

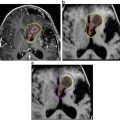
Fig. 2
Body mass index (BMI) and magnetic resonance imaging (MRI) at diagnosis and 36 months after surgery in three cases of childhood-onset adamantinomatous craniopharyngioma with different grade of hypothalamic involvement/lesion. (a, b) Patient with craniopharyngioma confined to the intrasellar space [0° = no hypothalamic involvement (a)/surgical lesion (b)]. BMI at diagnosis: −0.11 SD; BMI 36 months after complete resection: −0.41 SD. (c, d) Patient with craniopharyngioma involving the anterior hypothalamus [Io = hypothalamic involvement (c)/surgical lesion of the anterior hypothalamic area (d)]. BMI at diagnosis : −1.75 SD; BMI 36 months after complete resection: −0.43 SD. (e, f) Patient with craniopharyngioma involving the anterior and posterior hypothalamus (IIo = hypothalamic involvement (e)/surgical lesion of the anterior and posterior hypothalamic area (f)]. BMI at diagnosis: +6.08 SD; BMI 36 months after complete resection: +6.79 SD. Arrows indicate mammillary bodies, defining the border between anterior and posterior involvement/lesion. Reproduced from Muller et al. [43] with kind permission of Bioscientifica
All of the above-mentioned treatment strategies and algorithms recommend that (a) for childhood-onset adamantinomatous craniopharyngioma with hypothalamic involvement, limited surgical approaches and postoperative external irradiation are advisable, and (b) treatment of craniopharyngioma should be confined to experienced multidisciplinary teams.
A major step towards potential standardisation of preoperative staging in childhood-onset craniopharyngioma is the comparison of published grading systems for assessment of hypothalamic damage/involvement in regard to prediction value for severe hypothalamic obesity as the main sequelae impairing quality of survival. Mortini et al. [48] have published one of the first studies which identify radiological variables linked to hypothalamic involvement on preoperative MRI, and correlate them with clinical features, long-term outcome, and prognosis. Additionally, Mortini et al. [48] analyzed the sensitivity of three published grading systems [40, 42, 43, 45] for prediction of hypothalamic obesity in their single centre cohort. Hypothalamic hyperintensity in T2-weighted/FLAIR images, unidentifiable pituitary stalk, dislocated chiasm, mammillary body involvement, retrochiasmatic tumour extension, and either infundibular recess or unrecognisable supra-optic recess have proved to be useful to define the hypothalamus invasion. Variables identified as factors with high and comparable prediction value for postoperative hypothalamic syndrome were the degree of hypothalamic involvement according to the classification described by Sainte-Rose and Puget [40] (p < 0.002; grade 0 vs 2 p < 0.001), Van Gompel et al. [45] (p < 0.002; grade 0 vs 1, p < 0.027; and grade 0 vs 2, p < 0.002), and Muller et al. [42, 43] (p < 0.006; grade 0 vs 1, p < 0.05; and grade 0 vs 2, p < 0.004). These results support the hypothesis that disease or treatment-related hypothalamic alterations have relevant negative impact on quality of survival and prognosis in childhood-onset adamantinomatous craniopharyngioma [49, 50].
There are only a few studies analysing the prognosis of patients with childhood-onset adamantinomatous craniopharyngioma in relation to the neurosurgeons’ experience [42, 43, 51–54]. Sanford [51] and Boop [52] reported clinically significant differences in outcome according to the neurosurgeons’ experience with the condition. Degree of obesity and quality of life were analyzed in a recent report based on reference assessment of tumour location and post-surgical hypothalamic lesions [43]. Treatment was also analyzed regarding neurosurgical strategy and the neurosurgical centre sizes based on patient load. Surgical lesions of anterior and posterior hypothalamic areas were associated with post-surgical obesity, negatively impacting long-term quality of survival in patients with surgical posterior hypothalamic lesions (Fig. 3). Treatment strategies in large centres were less radical and the rates of complete resection and hypothalamic surgical lesions were lower than those of middle and small-sized centres [42, 43, 54]. However, in multivariable analysis preoperative hypothalamic involvement was the only independent risk factor for severe obesity [43].
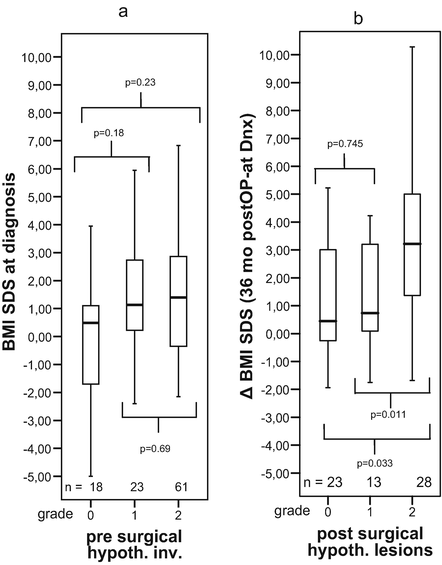

Fig. 3
Body mass index (BMI SDS ) at diagnosis (a) and increases in BMI (ΔBMI SDS) during 36 months after surgery (b) in relation to presurgical hypothalamic involvement (a) and surgical hypothalamic lesions (b) of 117 childhood-onset adamantinomatous craniopharyngioma patients recruited in KRANIOPHARYNGEOM 2000. The horizontal line in the middle of the box depicts the median. Edges of box mark the 25th and 75th percentile. Whiskers indicate the range of values that fall within 1.5 box-lengths. Reproduced from Muller et al. [43] with kind permission of Bioscientifica
Based on the current literature, it is advisable to have a multidisciplinary team able to discuss diagnostic and treatment strategies, adopting the most sophisticated approaches feasible based on sufficient in-house surgical, radiooncological and psychosocial experience for treating patients with childhood-onset craniopharyngioma [3, 8, 49, 55].
Radiooncological Treatment
The site and rate of progression of childhood-onset adamantinomatous craniopharyngioma, as well as the patient’s age, are important considerations when deciding whether reoperation and/or irradiation should be performed. Childhood-onset craniopharyngiomas are usually sharply bordered in the imaging. In contrast to primary brain tumour s, craniopharyngiomas tend towards less infiltrative growth, permitting a small safety margin of 5 mm maximum [56, 57]. Biological characteristics usually allow the option of using high-precision, three-dimensional conformation technology. A conventional, fractionated irradiation total target volume dose of 54 Gray has been established worldwide [56] [58].
Fitzek et al. published the first series of 15 craniopharyngioma patients treated with combined proton-photon irradiation for residual or recurrent disease [59]. Five- and 10-year local control rates were 93% and 85%, respectively, with 10-year survival expectancy in 72% of patients. Functional status, academic skills, and professional abilities were unaltered after proton beam therapy; no treatment-related neurocognitive deficits were reported. Luu et al. published a preliminary report on 16 patients treated with proton beam therapy [60]. Local tumour control could be achieved in 87% of patients. Late sequelae included newly diagnosed panhypopituitarism, an out-of-proton-field posterior fossa meningioma (59 months following proton beam therapy administered to patient who previously received photon radiotherapy), and a cerebrovascular accident. One study of proton beam therapy in craniopharyngioma reported on cyst growth during the treatment course: 24% of patients demonstrated cyst enlargement and 5% cyst reduction requiring modification of the treatment plan, while one patient required cyst drainage during treatment [61].
Clinical outcome data are still limited in proton beam therapy compared to modern photon therapy. However, proton beam therapy has the advantages of better dose conformation to the target volume, sparing of critical structures, reduced integral dose, and lower dose of secondary neutrons, which should reduce the risk of secondary malignancies [62, 63]. Data should become available in due course with sufficient follow-up time and as more centres are able to deliver proton beam therapy.
Instillation of Sclerosing Substances for Cystic Tumours
A catheter insertion into a cystic craniopharyngioma may prevail over the transient success of a cyst fenestration by allowing repetitive drainage of the craniopharyngioma cyst and intracystic instillation of sclerosing substances. Different neurosurgical techniques are employed for catheter placement. Even though it often results in only transient relieve of pressure, it is a useful therapeutic method for cystic recurrent tumours whose anatomical location and configuration makes them difficult to resect [64]. Instillation of sclerosing substances into craniopharyngioma cysts such as bleomycin, using an intracystic catheter implanted by an open or stereotactic procedure, was used in such cases [65]. Severe neurotoxic side effects were observed in cases due to cystic leakage of bleomycin into cerebrospinal fluid [66]. Accordingly, a thorough neuroradiological imaging for exclusion of cystic leakage is mandatory before instillation of bleomycin.
Intracystic instillation of interferon alpha was first used by Cavalheiro et al., who published the largest series on cystic childhood-onset craniopharyngiomas [67]. Their recent publication [67] included 60 children, who were treated at three different institutions between 2000 and 2009. Twenty-nine of the 60 patients received intracystic interferon alpha after initial surgery or bleomycin treatment had failed and 31 were treated with interferon alpha as a first line treatment. While in 47 children (78%) more than 50% cyst shrinkage was achieved, 13 children (22%) presented with progression and required surgical intervention. Only one-third of the patients experienced side effects consisting of either headaches, fever, chronic fatigue, palpebral edema, or arthritis not necessitating discontinuation of treatment. There was no mortality. Based on these reports on the effect and tolerability, intracystic instillation of interferon alpha is a promising therapeutic option for predominantly monocystic craniopharyngiomas [67, 68].
Long-Term Outcome and Morbidities
Pituitary Deficiencies
Pituitary hormone deficiencies are common in craniopharyngioma. At the time of diagnosis, 40–87% of children [34, 69–71] present with at least one hormonal deficit. 17–27% [34, 70, 72] have been reported to present with diabetes insipidus neurohormonalis. The rate of post-surgical pituitary hormonal deficits increases due to the tumour’s proximity or even involvement with the hypothalamic–pituitary axes [36, 38, 46, 69, 70, 72–74]. Transient post-surgical diabetes insipidus occurs in up to 80–100% of all cases [69, 75]. The rate of permanent post-surgical central diabetes insipidus ranges between 40 and 93% [38, 43, 69, 70, 72, 74–77].
In adult-onset craniopharyngioma patients anterior pituitary deficiencies and diabetes insipidus are most common and the majority of patients present with hypopituitarism [12, 71, 78, 79]. Endocrine deficiencies may worsen upon treatment. Mortini et al. [80] reported that 82%, 76%, 73%, and 67% of adult-onset craniopharyngioma patients with normal baseline levels for GH, ACTH, TSH, and gonadotropins developed a new deficits of the respective pituitary axes after surgery. Post-surgical onset of DI was observed in 70% of their patients. After transsphenoidal surgical approach the risk for new endocrine deficits appears to be lower [71, 80]. Recovery of preexisting pituitary dysfunction after surgery is rather rare. Most adult-onset craniopharyngioma patients suffer from partial or complete pituitary deficiencies as well as diabetes insipidus, with approximately 80% requiring the substitution of more than two pituitary hormones [12, 81].
Growth hormone deficiency has been reported at the time of diagnosis in 26–75% of childhood-onset craniopharyngioma patients [34, 77], and impaired growth, one of the early clinical manifestations of craniopharyngioma, often occurs years before diagnosis [36]. Growth hormone deficiency following tumour treatment for childhood-onset craniopharyngioma is found in about 70–92% of patients [36, 43, 82, 83] and a positive response to growth hormone treatment is seen in most cases [84]. Normal growth rates in childhood-onset craniopharyngioma patients with proven growth hormone deficiency are reported in the literature [85]. In fact, childhood-onset craniopharyngioma patients with hypothalamic involvement were found to achieve normal adult height more often than those without hypothalamic involvement [36]. Even though this phenomenon of “growth without growth hormone” was described in childhood-onset craniopharyngioma five decades ago [86], the physiology of growth in these cases is still not fully understood, although insulin and/or leptin are suspected to play a compensating role in this context. Both insulin and leptin have been hypothesised to induce growth in the fetus and in obese children [87–89], with leptin reported to function as a bone growth factor acting directly at the level of skeletal growth centres, independently of growth hormone action [87]. Mechanisms by which insulin stimulates growth include its known anabolic effects . At high serum concentrations, insulin may bind to the type 1 insulin-like growth factor (IGF) receptor and induce growth, mediated by its effect to decrease IGF-binding protein 1 levels, resulting in increased levels of free IGF-1 [87]. In support of this theory, obese childhood-onset craniopharyngioma patients were found to present with higher height SDS at the time of diagnosis and at last follow-up with no difference in endocrine substitution, including growth hormone treatment [31]. In contrast, another study found that childhood-onset craniopharyngioma patients who were growing despite growth hormone deficit had the same body composition, anthropometrical measures, and metabolic indexes, including insulin levels, as those patients requiring growth hormone substitution [85].
Visual and Neurological Outcomes
Due to frequent suprasellar tumour location, visual deficits (both visual acuity and visual fields) are relatively common in patients with craniopharyngioma: visual impairment as an initial clinical manifestation of craniopharyngioma is found in more than half of the affected patients [34], with some post-surgical improvement of vision in 41–48% of patients [69, 76]. Risk factors for post-surgical visual impairment include prechiasmatic tumour location and severe pre-surgical visual deficits [46, 69]. Improved ophthalmological outcome has been detected in surgical cases using the transsphenoidal approach [76], but such an approach is limited to resection of mainly intrasellar tumours. Because most childhood-onset adamantinomatous craniopharyngiomas typically extend to the suprasellar area, they are best removed through a transcranial or a combined transcranial and transsphenoidal approach.
Neurological sequelae include epilepsy, hemiparesis, cranial nerve deficits, and cerebrovascular disease manifestations [72, 74, 83]. Most of these sequelae are transient and the total prevalence of long-term neurological complications is reported to be 8% [69], but increases to 36% for large-sized tumours [72] and 30% when including both visual and neurological complications [75].
Morbidity Related to Treatment Strategies and Hypothalamic Injury
For favourably localised craniopharyngiomas , the preferred treatment of choice, especially at initial diagnosis, is an attempt at complete resection with preservation of hypothalamic and visual function [3, 4, 40, 53, 90–94]. For unfavourably localised tumours—those too close to or too entangled with the hypothalamus and/or the optic chiasm—a limited resection should be considered in order to preserve integrity of and/or to avoid further damage to optic and hypothalamic structures [5, 54, 95–101]. Endoscopic routes provide novel and less traumatic approaches for surgical resection [102–104].
The implantation of an intracystic catheter with a subcutaneous Ommaya reservoir enables the possibility of repeated decompression of the cyst and instillation of sclerosing agents [105, 106]. Bartels et al. [68] report high efficiency and good tolerability of interferon alpha as an intracystic sclerosing agent.
Irradiation is effective in preventing progression of residual tumour and relapses and is therefore a recommended treatment option in cases of limited surgical perspectives [107, 108]. First experiences with proton beam therapy applied to childhood-onset adamantinomatous craniopharyngioma are promising, offering a more protective radiooncological option than conventional photon irradiation, especially for tumours localised in the vicinity of hypothalamus or optic chiasm [109]. Stereotactic irradiation and gamma knife treatment are options in rare cases [110, 111].
Hypothalamic Obesity
Symptoms related to hypothalamic syndrome , such as obesity, behavioural changes, disturbed circadian rhythm, daytime sleepiness, and imbalances in regulation of thirst, heart rate, body temperature, and/or blood pressure, have been found at diagnosis in approximately 35% of childhood craniopharyngioma patients [3, 4, 10, 112–115]. The rate of hypothalamic dysfunction dramatically increases after treatment of craniopharyngioma; in some series up to 65–80% [5].
Suprachiasmatic lesions are associated with high morbidity. Surgical removal of sellar masses with suprasellar extension beyond the mammillary bodies risks hypothalamic structures and consequent hypothalamic syndrome with consecutive obesity [40, 42, 95, 116–118]. With the aid of imaging studies, recent reports have shown that the degree of obesity of affected patients is positively correlated with the degree of hypothalamic lesions [40, 42, 43, 119]. Based on this observation, novel classifications of pre-surgical involvement and post-surgical damage of hypothalamic structures based on MRI have been recently published [42, 43, 95] that might help to establish risk-adapted, i.e. hypothalamus-sparing surgical strategies .
Weight gain in childhood-onset adamantinomatous craniopharyngioma frequently occurs years before diagnosis [36], with 12–19% of patients reported to be obese at the time of diagnosis [4]. Weight gain and the development of obesity occur despite adequate endocrine replacement of pituitary deficiencies. The hypothalamic imbalances in energy management contribute to obesity and are exacerbated by factors limiting physical activity such as marked daytime sleepiness [120] and visual impairment. The degree of obesity frequently increases early after initial surgical treatment and rapid weight gain occurs the first 6–12 months after neurosurgical treatment [36]. Following initial treatment, the prevalence of severe obesity is higher, reaching up to 55% [49]. Severe obesity results in increased risks of cardiovascular disease and sequelae due to metabolic syndrome and [116].
The relation of severe obesity with hypothalamic lesions is obvious in childhood-onset adamantinomatous craniopharyngioma [7, 121, 122]. It is likely that in cases of craniopharyngioma with suprasellar extension, hypothalamic function will be compromised and will remain compromised after surgical and/or radio-oncological treatment. The hypothalamus contains several groups of nerve cell bodies forming distinct nuclei, which have highly diverse molecular, structural and functional organisations [121, 123]. The hypothalamus plays a dominant role in keeping the internal environment stable by synchronising circadian rhythms. Recent data indicate that appropriate balance of the autonomic nervous system has major impact on metabolism [124]. It is well known that adipose tissue is richly innervated by sympathetic nerve fibres controlling lipolysis. It now appears that lipogenesis is also controlled by parasympathetic innervation of adipose tissue originating from separate parasympathetic and sympathetic neurons located in the suprachiasmatic (SCN) and periventricular nucleus [122–124]. Such a high level of differentiation puts the SCN in a major position to balance circadian activity of both sympathetic and parasympathetic branches of the autonomous nervous system [122]. Considering the large proportion of craniopharyngioma patients with damage to suprasellar structures, it is most likely that craniopharyngiomas and/or the effects of treatment damage the SCN. This in turn influences the regulation of central clock mechanisms, which predisposes to metabolism alterations [120]. Accordingly, surgical strategies aiming at preservation of hypothalamic integrity are mandatory for the prevention of severe obesity owing to hypothalamic damage [3].
A study of Harz et al. [125] involving self-assessment by nutritional diaries showed that hypothalamic obesity occurs in patients with childhood-onset craniopharyngioma even though their caloric intake is similar to BMI-matched controls. The analysis of physical activity using actimetry showed that childhood-onset craniopharyngioma patients presented with significantly lower levels of physical activity than BMI-matched healthy controls [125]. The aforementioned disturbances of circadian rhythms and marked daytime sleepiness have been demonstrated in patients with childhood-onset craniopharyngioma and hypothalamic obesity [120], which in turn were correlated with low, early morning and nocturnal melatonin concentrations in saliva [126, 127]. The proposed pathogenic mechanism involves impaired hypothalamic regulation of circadian melatonin secretion rhythms in patients with childhood-onset craniopharyngioma extending to suprasellar areas. Initial experiences with melatonin substitution in childhood-onset craniopharyngioma patients were promising: daytime sleepiness and physical activity improved and melatonin levels normalised [126].
Polysomnographic studies in patients with childhood-onset adamantinomatous craniopharyngioma and severe daytime sleepiness have revealed sleeping patterns typical for hypersomnia and secondary narcolepsy, i.e. frequent “sleep-onset REM phases” (SOREM) [120, 128, 129]. Medication with central stimulating agents (methylphenidate, modafinil) had a markedly beneficial effect on daytime sleepiness in these patients [129]. Secondary narcolepsy should be taken into consideration as a pathogenic factor in severely obese childhood-onset craniopharyngioma patients. Mason et al. [130] treated five patients with childhood-onset craniopharyngioma and severe hypothalamic obesity (age range: 6.0–9.8 years) with the central stimulating agent, dextroamphetamine, for the purpose of weight reduction. Dextroamphetamine therapy stabilised patient body mass index. The patients’ parents reported noticeable improvements in their child’s physical activity and alertness. Several reports [124, 131] hypothesised that decreased physical activity and severe obesity in patients with childhood craniopharyngioma are likely related to impaired central sympathetic output. For instance, Roth et al. [132] observed reduced urine concentrations of catecholamine metabolites correlating with the degree of obesity and the level of physical activity.
A decreased metabolic rate, in terms of both total and resting energy expenditure, contributes to the development of severe obesity in this population. Pediatric and adult patients with childhood-onset adamantinomatous craniopharyngioma were found to have a lower resting energy expenditure (REE) when compared to controls [133]. The energy intake/REE ratio was lower in patients with tumour involvement of the third ventricle [134]. Further factors that could potentially contribute to decreased physical activity are visual and neurological deficits [135, 136], psychosocial difficulties [49], and increased daytime sleepiness [120, 126].
Roemmler-Zehrer et al. [137] recently reported on the gastrointestinal hormones peptide YY and ghrelin and their effect on satiety in obese craniopharyngioma patients. Their findings support the hypothesis that reduced ghrelin secretion and reduced postprandial suppression of ghrelin and severe obesity lead to disturbances of appetite regulation in craniopharyngioma patients. However, peptide YY levels did not differ between normal weight, obese, and very obese patients. Further potential pathogenic roles of brain-derived neurotrophic factor and peripheral α-melanocyte-stimulating hormone in childhood-onset adamantinomatous craniopharyngioma obesity have been postulated [138, 139].
When elevated serum leptin levels relative to body mass index were found in childhood craniopharyngioma patients with a suprasellar tumour extension [140], researchers suggested that normal appetite inhibition failed to occur in these patients due to disruption of hypothalamic receptors that regulate the negative feedback loop in which leptin, formed in adipocytes, binds to hypothalamic leptin receptors.
Hoffmann et al. [141] recently reported on non-alcoholic fatty liver disease (NAFLD) , a severe, previously underestimated sequelae in childhood-onset craniopharyngioma patients with hypothalamic obesity. NAFLD occurred in about 50% of childhood-onset craniopharyngioma patients with hypothalamic obesity and was associated with elevated liver enzymes and HOMA index. Over half of all patients (60%) with NAFLD were treated by stimulating agents for hypersomnia and increased daytime sleepiness as clinical manifestations of secondary narcolepsy, exerting considerable liver toxicity. The authors recommended that stimulating agents for treatment of secondary narcolepsy in childhood-onset adamantinomatous craniopharyngioma should be prescribed judiciously.
Challenges in Treating Hypothalamic Obesity
Due to the above-reported disturbances in energy expenditure, appetite regulation, and central sympathetic output, craniopharyngioma patients with hypothalamic involvement typically develop morbid obesity that is mainly unresponsive to conventional lifestyle modifications [3, 4, 14, 93, 123, 142, 143].
Recent studies on novel pharmaceutical treatment options for hypothalamic obesity in craniopharyngioma patients report mixed results [10].
Sibutramine is a neurotransmitter reuptake-inhibitor that reduces the reuptake of serotonin, norepinephrine, and dopamine, thereby increasing the levels of these substances in synaptic clefts and helping enhance satiety. Sibutramine has been widely used to treat obesity, leading to a weight loss of 7–10% when combined with a regulated diet. Sibutramine was tested in a randomised placebo-controlled crossover trial in patient cohorts with different obesity syndromes [144]. While the drug was well tolerated and safe, the weight loss response in patients with hypothalamic obesity was less pronounced in comparison to other participants (such as trisomy 21 and Prader–Willi syndrome). While the effect on body mass index was promising, sibutramine has been taken off the market due to adverse side effects and further clinical trials are not expected.
Based on impairment of epinephrine production and sympatho-adrenal activation manifesting as reduced hormonal response to hypoglycaemia, treating this disorder with amphetamine derivatives has been suggested [145]. In a prospective open-label 6-month pilot treatment trial in nine obese subjects with childhood-onset craniopharyngioma Hamilton et al. [146] assessed the effect of combined diazoxide-metformin therapy . Combined diazoxide-metformin therapy was associated with reduced weight gain in patients with hypothalamic obesity. Insulin levels at study commencement predicted effectiveness of the treatment. Kalina et al. [147] analysed the effect of fenofibrate and metformin treatment on metabolic status in 22 childhood-onset craniopharyngioma patients. The authors reported a positive effect on homeostatic model assessment (HOMA) and dyslipidemia . Zoicas et al. [148] treated eight adult patients with hypothalamic obesity (six craniopharyngioma) with GLP-1 analogues and observed a substantial and sustained weight loss associated with improvements in cardiovascular and metabolic risk profiles. Van Santen et al. [149] reported a case with hypothalamic obesity resulting from treatment of craniopharyngioma in which T3 mono-therapy was not effective in increasing REE or brown adipose tissue (BAT) activity. This may be explained by damage to the ventromedial hypothalamic region, which is a key area in the hypothalamus for T3-mediated BAT activation . Whereas substitution therapy with recombinant growth hormone is efficient in promoting normal growth, clinically relevant weight reducing effects are not observed in childhood-onset patients with hypothalamic obesity [150, 151].
Childhood-onset craniopharyngioma patients with hypothalamic obesity have a parasympathetic predominance of the autonomic nervous system induced by vagal activation, manifesting as reduced heart rate variability and daytime sleepiness [124]. Parasympathetic stimulation induces insulin secretion by direct β cells activation as well as adipogenesis. Insulin is an anabolic hormone and an important driver of weight gain in hypothalamic obesity. Octreotide as a somatostatin analogue reduces insulin secretion. Lustig et al. [152] tested octreotide in a double blind randomised controlled study in children with hypothalamic obesity, demonstrating moderate reductions in weight gain in which insulin levels during a proof-of-concept oral glucose tolerance test decreased without leading to relevant changes in glucose tolerance.
Initial experiences with laparoscopic gastric banding (LAGB) in severely obese childhood-onset adamantinomatous craniopharyngioma patients achieved sufficient tolerability and short-term weight reduction [153–155]. An instant improvement of binge-eating behaviour in craniopharyngioma patients was observed immediately after bariatric procedure (LAGB) [155], but failed in long-term weight reduction. Nevertheless, weight stabilisation could be achieved with regular follow-up monitoring [156]. In a systematic review and meta-analysis of the literature, Bretault et al. [153] analyzed the 12 months outcome after bariatric surgery for hypothalamic obesity due to craniopharyngioma treatment. At 1 year, among 18 cases with follow-up data, six had lost more than 20% of their initial weight; all had undergone either Roux Y gastric bypass (n = 3), sleeve gastrectomy, (n = 2), or biliopancreatic diversion (n = 1). All patients who had lost less than 5% of their initial weight had undergone LAGB except one Roux Y gastric bypass case. These findings indicate that Roux Y gastric bypass , sleeve gastrectomy, and biliopancreatic diversion are the most efficient bariatric procedures for weight reduction in hypothalamic obesity of childhood craniopharyngioma. However, treatment with invasive, non-reversible bariatric methods are controversial in the pediatric population because of medical, ethical, and legal considerations [156–158].
Reports on tolerability and efficacy of gastric pacemaking devices [159] and deep brain stimulation [160] in treatment of hypothalamic obesity have not been published in craniopharyngioma patients.
Despite the availability of promising therapeutic approaches [161], it must be emphasised that currently no generally accepted therapy for hypothalamic obesity in craniopharyngioma has been shown to be effective in randomised studies. Furthermore, structured rehabilitation programs for weight reduction in survivors of childhood-onset adamantinomatous craniopharyngioma have no proven persistent long-term effect on weight stabilisation or reduction [162].
Eating Behaviour
Relevant associations between obesity and an obesogenic environment and consequent eating behaviour have been confirmed in children and adolescents [7, 10]. Due to the dearth of studies on eating behaviour in childhood-onset adamantinomatous craniopharyngioma patients, Hoffmann et al. [165] analysed eating disorders and eating behaviour in 101 survivors of childhood-onset craniopharyngioma and 85 BMI-matched healthy controls. Severely obese patients (BMI >+8 SD; n = 9) presented with pathological eating behaviours and more weight problems and eating disorders, as compared to obese (BMI +3 to +8 SD; n = 44) and normal or overweight patients (BMI <+3 SD; n = 48). However, childhood-onset craniopharyngioma patients with different degree of obesity showed similar or even less pathological findings as compared to BMI-matched normal controls. Hoffmann et al. conclude that the observed eating disorders are not disease-specific for childhood-onset craniopharyngioma.
Roth et al. [166] assessed pre- and post-meal responses to visual food cues in childhood-onset adamantinomatous craniopharyngioma patients’ brain regions of interest using functional magnetic resonance imaging (fMRI). Following a test meal, BMI-matched controls showed suppression of activation by high-calorie food cues while childhood-onset craniopharyngioma patients showed trends towards higher activation. These findings support the hypothesis that perception of food cues especially after food intake may be altered in childhood-onset craniopharyngioma patients with hypothalamic obesity.
Roemmler-Zehrer et al. [167] compared eating behaviour in 26 craniopharyngioma patients (four childhood-onset cases) with 26 patients with non-functioning pituitary adenoma. Whereas craniopharyngioma patients scored higher in terms of conscious hunger perception, the rate of eating disorders was similar in both groups, supporting the hypothesis that eating disorders in patients with hypothalamic obesity are not disease specific.
Even though hypothalamic obesity is a frequent sequela in childhood-onset adamantinomatous craniopharyngioma [168], also diencephalic syndrome leading to severe weight loss and cachexia can occur as a rare hypothalamic disturbance of body composition in childhood-onset craniopharyngioma [169, 170]. Hoffmann et al. [170] analysed the incidence of diencephalic syndrome, its clinical manifestations before and after diagnosis of childhood-onset craniopharyngioma, and outcome in 485 patients recruited in the German childhood craniopharyngioma registry. Only 4.3% of all childhood-onset craniopharyngioma patients presented with low weight (BMI <−2 SD) at time of diagnosis. Initial significant differences between patients with low weight at the time of diagnosis and normal weight patients at diagnosis are usually observed at 5 years of age. Within the first 2 years after diagnosis of childhood-onset craniopharyngioma, the BMI of diencephalic syndrome patients and normal weight patients converge to a similar level. Hoffmann et al. concluded from their analysis of patients’ histories that diencephalic syndrome at the time of diagnosis does not preclude subsequent weight gain caused by a childhood-onset adamantinomatous craniopharyngioma with hypothalamic involvement.
Sequelae, Prognosis, and Quality of Life
Quality of Life, Neurocognitive Outcome, and Psychosocial Functioning
Quality of life in childhood-onset craniopharyngioma patients can be affected by both the tumour itself and the treatment received [171]. Reports assessing physical and psychosocial functioning show variable results, ranging from excellent in a majority of subjects to impaired function in almost half of the patients [70, 75, 172–175]. The most common areas of impairments reported include emotional and social functioning, with patients rating their psychosocial status to be lower than their physical health [75]. Other reported challenges include somatic complaints such as pain, reduced mobility, and self-care [74, 75]. Behavioural studies indicate a high rate of psychopathological symptoms, including depression, anxiety, and withdrawal. The most frequent problems in children’s everyday functional capacity include inability to control emotions, difficulties in learning, unsatisfactory peer relationships, and concerns regarding physical appearance and body image [83, 176].
Reported factors associated with impaired quality of survival outcomes as well as psychosocial and neurocognitive functioning include younger age at the time of diagnosis and preoperative functional impairment. Furthermore, tumour characteristics —including larger tumour volume, hypothalamic, and 3rd ventricle involvement at presentation—are reported in the literature to complicate both pre- and post-surgical conditions and therefore survival and quality of life outcomes in these patients [38, 42, 49, 177]. Furthermore, treatment strategies have been implicated with worse outcomes for surgery alone compared to limited surgery followed by irradiation and for multiple operations for tumour recurrence . Neurological, endocrine, and ophthalmological sequelae all adversely affect quality of life outcome [46, 70, 72, 74, 75, 172, 173]. Hypothalamic dysfunction has been found to have the most important negative impact on physical ability, body image, and social functioning [31, 75, 173].
Long-term neurocognitive complications following treatment for craniopharyngioma include cognitive problems, particularly those affecting attention, executive function, episodic memory, and working memory [75, 83, 176, 178–182]. Özyurt et al. [183] observed that childhood-onset craniopharyngioma patients had lower performance scores in tests of memory and executive functioning when compared with normal controls. Performance in functional capabilities and executive functions were negatively associated with the degree of hypothalamic involvement and damage.
Long-term survivors of childhood craniopharyngioma treated primarily with subtotal surgical resection followed by irradiation were also found to have significant psychological and educational deficits [83]. Neurocognitive deficits include slower cognitive speed, attention problems, memory disturbances, and behavioural instability [83, 176, 178, 180, 181, 184]. Whereas intact intellectual functioning has been reported in up to 82% of patients, visual memory is impaired despite normal visual-spatial abilities [83, 176]. The observed deficits in higher cognitive processing such as attention problems are considered precursors to poor academic achievement.
Despite over a quarter of century of literature documenting the neurocognitive challenges encountered by patients treated for craniopharyngioma, intervention efforts have lagged. Recent case studies have examined the efficacy of cognitive rehabilitation for dysexecutive problems and behavioural lability [185, 186]. In a case report of a 2-month intervention using a combination of goal management therapy and naturally occurring distractions within the patients’ work environment, significant improvements in cognitive tests requiring organised behaviour were reported. Emotional, social, and/or behaviour problems, most notably aggressive behaviour, have been reported [75, 179, 187]. Despite their occurrence, the assumption of the biological underpinning of behavioural disturbances in these patients appears to result in limited attempts to effectively manage these disturbances with intervention. Behavioural treatment was used for severe aggressive behaviours [186]; the intervention included extinction of dysfunctional conditioned responses and functional behavioural analysis followed by differential reinforcement of alternative behaviours, with the goal of decreasing the frequency of aggressive behaviour. Aggressive behaviour subsequently decreased to below 88% of baseline levels and adaptive behaviours were found to increase significantly. These results suggest that patients’ aggression is maintained by inadvertent social reinforcement. Taken together, these case studies suggest that cognitive rehabilitation efforts such as functional behavioural analysis and goal management therapy appear to be useful diagnostic and therapeutic options, compensating for psychosocial and cognitive challenges [188].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree