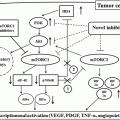
Characteristic
Temsirolimus
Everolimus
Study population
Treatment-naive patients with poor-risk disease
Patients who had progressed on prior sunitinib and/or sorafenib
Number of patients
626
410
Randomization
Temsirolimus vs. temsirolimus/IFN-α vs. IFN-α
Everolimus/BSC vs. placebo/BSC
Primary end point
OS
PFS
Met primary end point?
Yes
Yes
ΔPFS (P-value)a
1.9 mos (P = NR)
3.0 mos (P, 0.001)
ΔOS (P-value)a
3.6 mos (P = 0.008)
0.39 mos (P = 0.177)
The overall survival benefit achieved in the global ARCC trial led the US Food and Drug Administration (FDA) to approve temsirolimus on May 30, 2007, as an anticancer therapy for use in the first-line setting of advanced poor-risk RCC patients [20].
However, due to difficulties in the enrollment of pure poor-risk patients (defined, according to the MSKCC criteria, as patients having three or more of the following five negative prognostic characteristics: anemia, high LDH levels, high corrected calcium levels, poor Karnofsky performance status, and an interval between diagnosis and start of treatment of less than a year), the protocol was emended, with a sixth negative prognostic feature (i.e., multiple metastatic sites) added to the original MSKCC criteria to classify patients as poor risk [18]. Such a change allowed for the enrollment into the study also of patients from the intermediate MSKCC risk group.
Notably enough, a subgroup analysis of the study suggested that real poor-risk patients (i.e., those classified as such according to the original MSKCC criteria) benefited most for the treatment [19]; furthermore, patients with non-clear cell histologies seemed to achieve a preferential benefit from temsirolimus too [19].
11.4.4 Other Temsirolimus Phase III Trials in RCC: INTORSECT and INTORACT
More recently, temsirolimus was compared with the multikinase inhibitor sorafenib within the INTORSECT (Investigating Torisel as Second-Line Therapy) phase III trial, as a second-line therapy in patients with metastatic RCC after disease progression on first-line sunitinib [20]. As a whole, 512 RCC patients were randomly assigned to receive either temsirolimus, given i.v. at the dose of 25 mg once weekly (n = 259), or oral sorafenib at the standard dose of 400 mg twice per day, continuous dosing (n = 253), with stratification according to duration of prior sunitinib therapy (≤ or >180 days), prognostic risk, histology (clear cell or non-clear cell), and nephrectomy status. No significant PFS difference between treatment arms was observed, median PFS being 4.3 and 3.9 months in the temsirolimus and sorafenib arms, respectively (stratified hazard ratio [HR]: 0.87; 95 % CI, 0.71 to 1.07; p = .19). Surprisingly enough, a statistically significant (and clinically relevant) OS difference in favor of sorafenib was observed: 16.6 vs. 12.3 months (stratified HR: 1.31; 95 % CI, 1.05 to 1.63; p = .01).
Another phase III trial was designed and conducted to prospectively determine the efficacy of a combination of temsirolimus plus bevacizumab as compared to bevacizumab plus interferon-α in previously untreated advanced RCC patients: the INTORACT (Investigation of Torisel and Avastin Combination Therapy) study [21]. Patients were randomized to receive the combination of either temsirolimus (25 mg i.v. weekly) or interferon-α (9 MIU s.c. thrice weekly) with bevacizumab (10 mg/kg i.v. every 2 weeks), the primary end point being once again PFS. There were no significant differences in both PFS (9.1 and 9.3 months, respectively; HR: 1.1; 95 % CI, 0.9 to 1.3; p = .8) and overall survival (25.8 and 25.5 months, respectively; HR: 1.0; p = .6) with temsirolimus plus bevacizumab as compared to bevacizumab plus interferon-α, respectively. Despite differences in overall mean scores in the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI)-15 and FKSI-Disease-Related Symptoms subscales (in favor of the combination of temsirolimus plus bevacizumab), no differences in global health outcome measures were observed. Finally, treatment-emergent all-causality grade ≥ 3 adverse events were more common (p < .001) with temsirolimus plus bevacizumab and included mucosal inflammation, stomatitis, hypophosphatemia, hyperglycemia, and hypercholesterolemia, whereas neutropenia was more common in the control arm (i.e., bevacizumab plus interferon-α).
As the negative results of the INTORSECT trial hampered the development of temsirolimus in second or later treatment line in RCC [22], the same happened for Temsirolimus first-line use outside the setting of poor-risk patients.
11.5 Everolimus
Everolimus (RAD001) is the other mTOR inhibitor that has been developed for the treatment of advanced RCC; it is administered orally, on a continuous daily schedule (even though a weekly schedule has been also tested, especially for combination regimens and in indications different from RCC) [23].
11.5.1 Phase I Studies
Based on preclinical data with weekly treatment schedules [24], an initial phase I trial in advanced solid tumors explored both weekly and daily dosing of the oral formulations of the drug [23]. In the first phase, patients were treated with weekly doses ranging from 5 to 30 mg. No dose-limiting toxicities (DLTs) were observed, and accompanying correlative studies assessing peripheral blood mononuclear cells (PBMCs) showed downregulation of relevant downstream moieties (i.e., p70S6 K). In the second part of the study, patients were treated with weekly doses of everolimus above 30 mg and daily doses of 5 or 10 mg. DLT was seen in one patient each at 50 mg/week (stomatitis and fatigue) and 10 mg/day (hyperglycemia). Ultimately, it was determined that doses of 70 mg weekly and 10 mg daily could be satisfactorily tolerated. Although the half-life of everolimus (∼30 h) was thought to facilitate weekly dosing of the drug, it was observed that daily dosing could produce more sustained target inhibition in preclinical models [25].
11.5.2 Phase II Study in RCC
The first published phase II trial of everolimus (dosed at 10 mg/day) for metastatic RCC, conducted by Amato et al. enrolled 41 patients with predominantly clear cell disease who had received up to one prior systemic treatment [26]. Most patients (83 %) had been previously treated, mainly in the form of immunotherapy (61 %). With 57 % of patients progression free for ≥6 months and median PFS of 11.2 months (95 % CI, 1.7–36.2 months), the study met the prespecified criteria for further evaluation. In all, 24 of 37 evaluable patients experienced some degree of tumor reduction. Objective responses per independent assessment were mainly stable disease (SD), lasting for ≥3 and ≥6 months in 74 % and 58 % of patients, respectively, with an additional two patients achieving a partial response (PR). Considering all patients, median OS was 22.1 months (95 % CI, 1.4–36.4 months). Most adverse events (AEs) were of grade 1/2 severity, with no grade 4 AEs reported. The most common treatment-related grade 3 AEs were pneumonitis (n = 7, 18 %) and alanine aminotransferase elevation (n = 4, 10.3 %), followed by alkaline phosphatase elevation, hyperglycemia, and thrombocytopenia (n = 3 each, 8 %).
11.5.3 Pivotal Everolimus Phase III Trial in RCC: The RECORD-1 Trial
Everolimus was approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib and/or sorafenib, following the presentation of the results of the RECORD-1 (Renal Cell Cancer Treatment with Oral RAD001 given Daily) trial.
The pivotal RECORD-1 trial was a randomized (2:1), placebo-controlled, phase III study, in which RCC patients who had failed treatment with one or two previous VEGFR tyrosine kinase inhibitors (TKIs); notably enough, the majority of patients had also failed other previous treatments [27].
A total of 416 patients were enrolled and stratified according to the number of previous treatments (sorafenib or sunitinib [1 TKI] vs. sorafenib as well as sunitinib [2 TKIs]) and prognostic risk group. Patients were then randomized in the ratio of 2 to 1 to receive everolimus (given at the standard dose of 10 mg daily, per os) plus best supportive care (BSC) or to placebo plus BSC. After the second interim analysis, the study was terminated since the prespecified efficacy end point had been met [27]. Indeed, at the final trial analysis, everolimus proved able to significantly improve PFS when compared to placebo: 4.9 months vs. 1.9 months, respectively (HR: 0.33; 95%CI, 0.25–0.43; p < 0.001) [28]. As a whole, the results of the RECORD-1 trial are summarized in Table 11.1.
Regarding OS, the high percentage of patients who crossed over from the placebo to the active drug precluded any chance to observe a significant difference between the two arms, although a subsequent statistical analysis, used to correct the estimate of the effect of treatment taking into account the bias generated by crossover, showed an OS 1.9 times longer in favor of everolimus [28].
11.5.4 Global Open-Label Expanded Access REACT Study
Based on the results of RECORD-1, the RAD001 Expanded Access Clinical Trial (REACT) [32] was initiated to provide everolimus in advance of regulatory approval and commercial availability to patients with metastatic RCC in whom prior VEGF TKI therapy had failed and to enable collection of safety and efficacy data in a larger and more diverse population of patients with RCC. A total of 1367 patients from 34 countries received everolimus, given at the standard dose of 10 mg, once daily. As in the RECORD-1 phase III trial, patients from all MSKCC risk categories were eligible, and the inclusion criteria were broadened to include patients with metastatic RCC of any histology, measurable or non-measurable disease, and brain metastases. Safety findings and tumor responses were consistent with those observed in RECORD-1, with no new safety issues identified. The most commonly reported serious AEs were dyspnea (5.0 %), pneumonia (4.7 %), and anemia (4.1 %), and the most commonly reported grades 3/4 AEs were anemia (13.4 %), fatigue (6.7 %), and dyspnea (6.5 %). Best overall response was stable disease and partial response in 51.6 % and 1.7 % of the treated patients, respectively. Median everolimus treatment duration was 14 weeks.
11.5.5 Multicenter, Non-interventional, Observational CHANGE Study
The efficacy and safety of everolimus in routine clinical practice in Germany was evaluated in a prospective observational study in patients with mRCC of any histology in whom one prior anti-VEGF therapy (including TKIs as well as bevacizumab) had failed [33].
Median time to disease progression (TTP), defined as the time from first everolimus intake to disease progression from any cause, was 6.6 months (95 % CI, 5.0–8.8 months) in the safety population (n = 195), 7.0 months (95 % CI, 5.1–9.0 months) in the efficacy population (n = 165), and 7.1 months (95 % CI, 5.5–9.0 months) in patients of the efficacy population who previously received only one VEGF TKI (n = 121). The prolonged median TTP compared with the RECORD-1 study (i.e., 4.9 months) might have been a result of the higher percentage of patients who received everolimus as a pure second-line therapy (72 % vs. 21 % for RECORD-1). The most commonly reported AEs (any grade occurring in >5 % of the safety population) associated with everolimus were dyspnea (14 %), anemia (13 %), nausea (9 %), pain (9 %), and stomatitis (8 %). Overall, more than 75 % of physicians reported a positive assessment of tolerance to everolimus and a high adherence to therapy.
11.5.6 RECORD-3: A Sequential Trial of Sunitinib Followed by Everolimus or Vice Versa
RECORD-3 is a randomized, open-label, multicenter, phase II, non-inferiority trial aimed at assessing the efficacy and safety of first-line everolimus followed by second-line sunitinib versus first-line sunitinib followed by second-line everolimus for the treatment of patients with mRCC [34].
In this trial, 471 patients with both clear (85.6 %) and non-clear cell metastatic RCC were evenly randomized between the two treatment arms. A majority of patients (86 %) presented with favorable or intermediate prognoses. Median follow-up was 22.7 months. Just 53.7 % of the patients who discontinued first-line everolimus received second-line sunitinib, and 51.6 % of patients who discontinued first-line sunitinib did receive second-line everolimus. Median PFS was 7.9 versus 10.7 months (HR = 1.43), respectively, whereas median OS was 22.4 versus 32.0 months (HR = 1.24), suggesting a trend toward improved survival in the sunitinib first arm. Common treatment-emergent adverse events for first-line everolimus vs sunitinib, respectively, were stomatitis (53 % vs. 57 %), fatigue (45 % vs. 51 %), and diarrhea (38 % vs. 57 %).
11.5.7 RECORD-4: Everolimus in a Pure Second-Line Setting
Since RECORD-1 demonstrated clinical benefit of everolimus in patients with metastatic RCC previously treated with sunitinib, sorafenib, or both (although prior treatments were also permitted), more recently the phase II RECORD-4 study prospectively assessed everolimus in a purely second-line setting, after having been exposed to sunitinib, other anti-VEGF therapy, or cytokines [35]. Overall median PFS was 7.8 months, while it was 5.7 months after sunitinib, 7.8 months after other previous anti-VEGF therapy, and 12.9 months after previous cytokines. Total median OS was 23.8 months, the same figure observed after previous sunitinib, while it was 17.2 months after other previous anti-VEGF therapy, median OS having not been reached yet after cytokines. The results of the RECORD-4 study ultimately confirmed the activity of second-line everolimus after first-line sunitinib or other anti-VEGF therapies.
11.6 The “Fall of Gods”: The Checkmate-025 and Meteor Trials
After being for years one of the two standards of treatment for the second (and third) line of metastatic RCC [36], in the past 2 years, everolimus was the losing arm of two large randomized controlled, phase III trials which ultimately changed the treatment landscape in the second (and further) treatment line setting. Indeed, after the results of the nivolumab versus everolimus Checkmate-025 trial and of the cabozantinib versus everolimus METEOR trial, everolimus lost the status of standard treatment according to all the major international guidelines [37–39].
11.6.1 The Checkmate-025 Trial
Checkmate-025 was a randomized, open-label, phase III study comparing nivolumab (a fully humanized IgG4 isotype monoclonal antibody that inhibits PD-1 and thus restores anticancer immune responses) with everolimus in previously treated RCC patients [40]. A total of 821 patients with advanced clear cell RCC for which they had received previous treatment with one or two regimens of antiangiogenic therapy were randomly assigned to receive 3 mg/kg of nivolumab intravenously every 2 weeks or everolimus. The primary end point of this trial was OS, while the secondary end points included ORR and safety. The median OS was 25.0 months with nivolumab and 19.6 months with everolimus, a difference that was statistically significant and that translated into a reduction in the risk of death of 27 % in favor of nivolumab (HR for death = 0.73, 98.5 % CI = 0.57 to 0.93). However, median PFS did not significantly differ between nivolumab (4.6 months) and everolimus (4.4 months; HR = 0.88). As far as the ORR, it proved to be greater with nivolumab than with everolimus (25 % vs. 5 %).
Finally, nivolumab was also associated with quality-of-life improvement compared with everolimus [41].
11.6.2 The METEOR Trial
METEOR was a randomized, open-label, phase III trial aimed at evaluating the efficacy of cabozantinib (a multikinase inhibitor which targets all the three VEGFRs, AXL, as well as c-Met) as compared with everolimus in patients with RCC that had progressed after VEGFR-targeted therapy [42]. In this study, 658 patients were randomized to receive cabozantinib at a dose of 60 mg daily or everolimus. The primary end point was PFS, while secondary efficacy end points were OS and OR rate. Median PFS was 7.4 months with cabozantinib and 3.8 months with everolimus, representing a 42 % reduction in the risk of progression and/or death in favor of cabozantinib (HR = 0.58; 95 % CI = 0.45 to 0.75). The OR rate was 21 % with cabozantinib and 5 % with everolimus.
As far as OS, at the final survival analysis [43], median OS was 21.4 months (95 % CI = 18.7-not estimable) with cabozantinib and 16.5 months (14.7–18.8) with everolimus, a difference that proved to be statistically significant in favor of cabozantinib (HR = 0.66 [95 % CI = 0.53–0.83]).
11.7 The Safety Profile of mTOR Inhibitors in RCC
Adverse events observed in patients treated with mTOR inhibitors are fairly constant, irrespective of each specific indication. They include cutaneous and mucosal events (i.e., stomatitis and skin rash), pulmonary dysfunction (noninfectious pneumonitis), metabolic abnormalities (elevated blood levels of glucose, cholesterol, and triglycerides), as well as immune-related events (i.e., increased incidence of infections) [44].
Metabolic and immune-related adverse events are clearly on-target effects of mTOR inhibition, while cutaneous and mucosal effects may have a less direct association with mTOR inhibition, although inhibition of mTOR-mediated growth and tissue repair and/or immune dysregulation has been proposed to be a factor in mucosal epithelia with high turnover. As far as the risk of infections is concerned, we should not forget that mTOR inhibitors were first developed as immunosuppressive agents and are still widely used as such in the transplantation setting.
As a whole, the safety profile of both mTOR inhibitors, as it comes from the two registrative studies (i.e., ARCC and RECORD-1) performed in RCC, is summarized in Table 11.2.
Temsirolimus (n = 208) [18] | Everolimus (n = 274) [28] | |||
|---|---|---|---|---|
All grades | Grades 3/4 | All grades | Grades 3/4 | |
Pulmonary | ||||
Cough | 26 | 1 | 30 | 0.7/0 |
Dyspnea | 28 | 9 | 24 | 6.2/1.5 |
NIP | 2 | 1 | 9.9 | 2.6/0 |
Non-pulmonary | ||||
Stomatitis | 20 | 1 | 38 | 4.0/0.4 |
Asthenia | 51 | 11 | 33 | 2.6/0.7 |
Fatigue | NA | NA | 31 | 5.5/0 |
Diarrhea | 27 | 1 | 30 | 1.5/0 |
Rash | 47 | 4 | 29 | 1.1/0 |
Nausea | 37 | 2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||