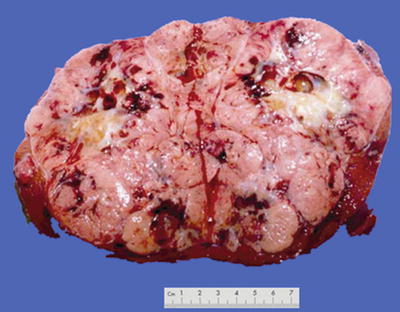
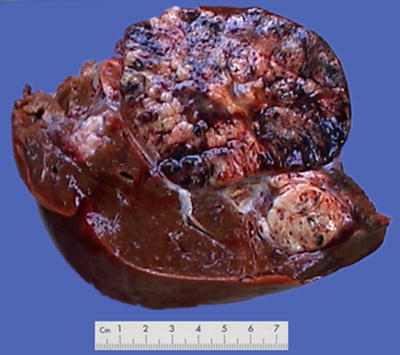
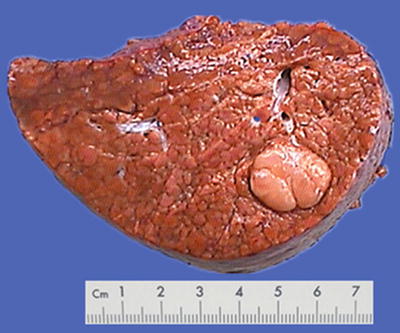
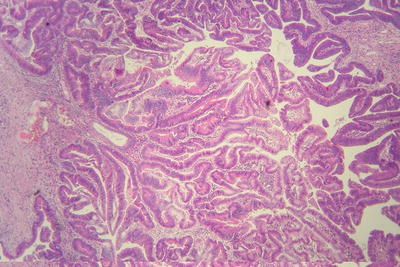
Fig. 7.2
Surgical resection of hepatobiliary tumors during the 30-year period in the EHBH
7.1.1.3 Pathogenesis and Mechanism
Currently, it is suggested that HCC relates to the following pathogenic factors:
- (1)
Infection of hepatitis viruses
- 1.
Infection of hepatitis B virus (HBV). According to the estimation by WHO, about two billion out of six billion of the world’s populations have infected with HBV, and 300 million people suffer from chronic HBV infection. In China, HCC with HBV infection is the most common epidemiological manifestations, and HBV infection is the main cause for 75–95% of HCC cases. Generally, patients with positive serum HBsAg for more than 6 months are known to have chronic HBV infection, and the risk of developing HCC in chronic HBV carriers is 200 times higher than that in non-HBV carriers. According to the national epidemiological survey on serum in 1992, HBV infection rate was about 60% in the population, with 700–800 million people infected with HBV, and the number of HBV carriers was about 120 million. However, the national epidemiological survey on serum epidemiology carried out by the Ministry of Health of China in 2006 demonstrated that HBsAg carrying rate in Chinese population was 7.18% and the carrying rate of HBsAg in children under 4 years old was lower than 1%. From the perspective of HBsAg carrying rate and HBV prevalence rate, the number of children infected with HBV in China had reduced by nearly 80 million people since 1992, with a reduction of nearly 19 million children carriers of HBsAg, and the national inoculation rate of hepatitis B vaccine had been raised from 39.95% in 1995 to 94.2% in 2005 according to the related investigations in China. And by 2010, both the incidence of hepatitis B and the rate of hepatitis B surface antigen carrying have decreased significantly, which is of great significance for the primary prevention of liver cancers and reduction in the incidence.
Based on the statistics of HCC patients who received surgical resection during the past 30-year period in the EHBH, the infection rate of HBV (either serum or immunohistochemical HBsAg positive) in these HCC patients was 85.86%. Due to the large population infected with HBV, the carrying rate of hepatitis B surface antigen in people aged from 15 to 59 years old was 8.57%. And according to the data released by Chinese Society of Hepatology, Chinese Medical Association, among about 93 million people with chronic HBV infection in China, there were 20 million patients, and the number of HBV infections would continue to increase in the future. Furthermore, 15–25% of people with chronic HBV infection develop into hepatic cirrhosis or liver cancer, which is the major cause responsible for the high incidence of HCC in China currently; thus, prevention and treatment of liver cancers are long and arduous tasks.
Researchers showed that the insertion of HBV-DNA gene into adjacent regions of the cancer gene or suppressor genes in the liver cells would cis-change the function of the gene resulting in disruption of related protein-encoding sequence. The integration of HBx gene into the host genome is the most crucial event, which can be found in genes of any length, and in 96% of the HCC specimens, all X genes are found to insert into the host cell DNA in the truncated form, suggesting trans-carcinogenic effects of the truncated X protein, such as exertion of the trans-transcription activity by the interaction between protein and protein. X protein encoded by HBx gene is a multifunctional regulatory protein and plays a key regulatory role in viral infection, replication, pathogenic, and carcinogenic processes. HBV integration results in a series of molecular variations, such as insertion mutation into the host genome, upregulated transcription driven by virus promoter, human-virus transcriptional fusion, variation of DNA copy number and induction of genomic instability, including activation of oncogenes (such as C-myc, KRAS, C-fos, IGF- II, IGF-IR) via trans-activation mechanism, inactivation of tumor suppressor genes (such as p53, RB, Bcl-2, DNA mismatch repair genes), regulation of epigenetic proteins via mediation of DNA methylation, or regulation of cell apoptosis, DNA damage and repair, cell cycle, and miRNA functions through transcriptional activation of various signal transduction pathways to promote the abnormal proliferation of liver cells leading to the formation of HCC, which is HBV-DNA integrated-type HCC. Recent studies demonstrated HBx can induce epithelium-mesenchymal transformation (EMT) via PI3K/AKT/GSK3β/Snail signaling pathway, promote the invasion and metastasis of HBV-HCC via formation of HBx-YAP conjugates [6, 7], or exert target inhibition of the function of tumor suppressor genes by regulating miRNA.
- 2.
Infection of hepatitis C virus (HCV). The global population of HCV infection reaches about 170 million, and there are about 35,000 new cases emerging each year. The Japanese HCV-related HCC account for 80–90% of all the cases. And the national serum epidemiological survey of China for virus hepatitis in 2006 showed that the prevalence rate of anti-HCV was 0.43% in Chinese population aged 1–59 years old, indicating that China was a low HCV endemic area in the global scope; however, the infection rate in China is increasing. It has been evaluated that the number of cumulative HCV infection in China exceeded 43 million. The serum anti-HCV positive rate in Chinese HCC patients is 6–32%, and the statistics of patients with HCC treated in the EHBH during the period of 30 years showed that HCV infection rate was 9.76%.
HCV is a single-stranded RNA virus encoding a single polyprotein precursor of about 3000 amino acids, generating more than ten proteins, among which NS5A protein, a transcription factor for cell growth, plays a key role in the canceration of liver cells through interaction with other proteins, such as participation in HCV protein mutation and replication of RNA, regulation of the expression of multiple genes in the host cells, stimulation of cell proliferation, inhibition of apoptosis, and reducing the curative effects of interferon [8]. Wurmbach et al. (2007) studied the signal pathways involving multiple stages in the development of HCV-related HCC and found dysregulation of several pathways, including Notch- and Toll-like receptor in the precancerous stage of hepatic cirrhosis, and several components of JAK/STAT pathway in the early stage of carcinogenesis, upregulation of expression of genes participating in DNA replication, and repair and cell cycle in late stage of carcinogenesis [9]. Because of no discovery of reverse transcriptase in the liver cells, HCV cannot integrate into the host genome but produce severe cytotoxic damage on the infected liver cells and modification of the host’s immune system through indirect interaction similar to oncogene proteins. In order to evade the host immune surveillance, HCV can constantly mutate to form variant strains after infection of host cells, resulting in constant degeneration necrosis, repeated regeneration, and proliferation of the host cells and finally causing chronic hepatic injury [10].
Generally, about 20% of the chronic HCV hepatitis will develop into hepatic cirrhosis, including 1–4% of the cases evolving into HCC. Therefore, the development from infection of HBV or HCV to HCC generally includes a stage ofchronic hepatitis and cirrhosis. Compared with HBV hepatitis, the period for chronic hepatitis HCV to develop into hepatic cirrhosis may be shorter. According to statistics of 26,330 cases of surgically excised HCC diagnosed in the Department of Pathology of EHBH, patients with cirrhosis accounted for 73% of all the cases. From the temporal perspective, the proportion of HCC cases with hepatic cirrhosis reached 95–100% before 2000, while it was 50–95% for HCC cases diagnosed after 2001, suggesting a potential increase in the number of non-cirrhotic HCC, and the pathological significance of this type of HCC needs further studies. For patients of chronic hepatitis or cirrhosis with persistent positive serum HBV/HCV, regardless of clinical symptoms and signs, physical examinations should be carried out regularly, including the monitoring of HCC markers, such as serum AFP, and standardized antiviral treatment should be adopted.
- (2)
Environmental pollution
- 1.
Food contamination aflatoxin (AFB1) is highly mutagenic, teratogenic, and carcinogenic and belongs to the first-degree carcinogens. High intake of AFB1 is associated with the pathogenesis of HCC in high-incidence area [11]. AFB1 can integrate with DNA in the liver cells to form AFB1-DNA adducts. The results of immunohistochemistry show that positive signals of AFB1-DNA adduct are located in the nuclei of the hepatocytes, the expression level of which is a biomarker reflecting the degree of AFB1 exposure. It can induce the transversion of arginine (AGG) to serine (AGT) on codon 249th of p53 gene in synergy with HBV, inducing gene mutation, or directly induce chromosome aberration. It has been shown that the exposure dose of AFB1 is 10–200 ng/kg in population in endemic areas of HCC, such as Southeast Asia, Africa, and Asia-Sahara area, while it is less than 3 ng/kg in the United States. Therefore, it is of importance to avoid intake of AFB1-contaminated food for the prevention of HCC.
- 2.
Drinking water contamination. The pollution of drinking water by substances, such as ammonia nitrogen, nitrite nitrogen, blue-green algae toxin, and humic acid, is closely related to the pathogenesis of HCC, and microcystin has a synergistic effect on the induction of HCC with chemical substances, such as AFB1.
- 3.
Environmental pollution. Chemical substances used in industries, such as vinyl chloride, phenol, arsenic, and aromatic amine compounds, have a strong induction effect on HCC.
- 1.
- (3)
Obesity and diabetes
According to a recent survey, children aged 7–13 years of age with an excess body mass index (BMI) have a significantly higher risk of HCC in their adulthood. An American study showed that patients with BMI ≥ 35 kg/m2 have a morality rate 4.5 times higher than that of the people with a normal BMI, and the relative risks of HCC in people with overweight and obesity are 117% and 189%, respectively. The risk of HCC developing in diabetic patients increases by three times compared to their nondiabetic counterparts [12]. The incidences of nonalcoholic fatty liver disease (NAFLD) in many countries have increased significantly, and it has become the second major liver disease after viral hepatitis. The cumulative incidence rate of HCC with NAFLD-related cirrhosis is 2.6%. And in the past 10 years, at least 300 cases of NAFLD with HCC have been reported in the literature, and defined risk factors for NAFLD include obesity, diabetes, hyperlipidemia, and insulin resistance. About 20% cases of NAFLD are complicated by fatty hepatitis, the latter of which is a risk factor for the development of cirrhosis and HCC. A German study showed that fatty hepatitis is an underlying cause in 24% of HCC patients, and average 55% of HCV patients in Western countries have NAFLD. In addition, there are also cases of HCC developed from non-cirrhosis NAFLD and nonfibrous fatty hepatitis.
- (4)
Genome variation
Mutations of cancer genes and/or inactivation of tumor suppressor genes (TSG) results in canceration of liver cells due to lack of regulation in signal transduction, cell cycle and growth, and proliferation by normal genes. The various molecular mechanisms and molecular types of HCC genomic variation are complex, and it has been found that human body has at least more than 1000 HCC-related genes with increasing discoveries of new HCC-related oncogenes, tumor suppressor genes, signal transduction pathways, and molecular targets. For example, He et al. (2011) conducted a genome-wide SNP microarray analysis on HCC tissues, screened out 1241 somatic copy number variation regions, and further identified 362 differentially expressed genes. They found that 60% of the HCC demonstrated lower expression >twofolds of TRIM35 gene which is a tumor suppressor and inhibits the proliferation of HCC cells. Higher expression (>twofolds) of HEY1 gene was found in 42.6% of the HCC, which is a cancer gene and promotes the proliferation of HCC cells [13]. Li et al. (2011) conducted a detection study on 18 thousand exons of coding genes using massively parallel sequencing technique, finding that 18.2% of HCV-HCC cases contain inactivating mutations of ARID2 gene, suggesting that it is a tumor suppressor gene for HCC [14]. A China-US joint research demonstrated a study using whole genome sequencing (WGS), and the results showed that β-catenin gene was the most common oncogene which mutate (15.9%) in HBV-HCC tissue, and TP53 gene was most susceptible to inactivation of anti-oncogenes (35.2%), while Wnt/beta-catenin pathway (62.5%) and JAK/STAT pathway (45.5%) were two signal pathways in which variations are the most frequently found for HCC [15].
Liu et al. (2014) investigated chromosomal DNA copy number changes in HCC by comparative genomic hybridization and detected the overexpression (>2-folds) of a new oncogene Maelstrom (MAEL) in 59.7% of the HCC cases. The experimental results showed that the MAEL is located on chromosome 1q24 and can activate Akt/ GSK-3β/Snail signaling pathway, inducing epithelial-mesenchymal transition (EMT) to promote the invasion and metastasis of HCC, and it also correlates with the recurrence and prognosis of these patients [16]. DLC-1 (deleted in liver cancer-1) gene is located on chromosome 8p21–22, encoding GTPase activator and can inhibit tumor metastasis. Deletion of DLC-1 gene has been found in 20% of HCC samples and 40% of HCC cell lines, and the growth of HCC cell strains transfected with DLC-1 was significantly inhibited, suggesting it is an inhibitory gene for HCC.
The biological characteristics of HCC are regulated by a complex cell signaling system, which are studied widely in aspects including the pathogenesis, growth, apoptosis, angiogenesis, invasion, metastasis, molecular therapeutic targets, and prognosis evaluation of HCC. And the representatives are tyrosine kinase pathway, Wnt/β-catenin pathway, P53 pathway, Notch pathway, NF-κB pathway, Hedgehog (Hh) pathway, Ras/Raf/MAPK pathway, VEGF pathway, JAK-STAT pathway, PI3K/Akt/mTOR pathway, HGF/c-Met pathway, and TGFβ1/Smads pathway, each of which is composed by diverse and complex key molecules. These pathways may be regulated by upstream miRNAs or target genes and exert the functions via the downstream target genes. Molecular analysis of molecules and function modes of HCC-related signal pathways is of clinical practice in aspects such as molecular typing, molecular diagnosis, and molecular-targeted therapy.
- (5)
Other factors
The association between alcoholic fatty liver disease (AFLD) and HCC has been recognized, and smoking can increase the risk of HCC development. And cases of HCC in patients with hereditary, congenital, allergic, or metabolic liver diseases, such as α1-antitrypsin protease deficiency, hereditary hemochromatosis, hereditary tyrosinemia, autoimmune hepatitis, primary biliary cirrhosis, have also been reported.
In a word, the genesis of HCC is a complex process involving multiple causes, mechanisms, steps, and genes. We briefly summarize the common causes of HCC, 16 key canceration mechanisms or research focus, and multistages during the pathological development (Fig. 7.3).
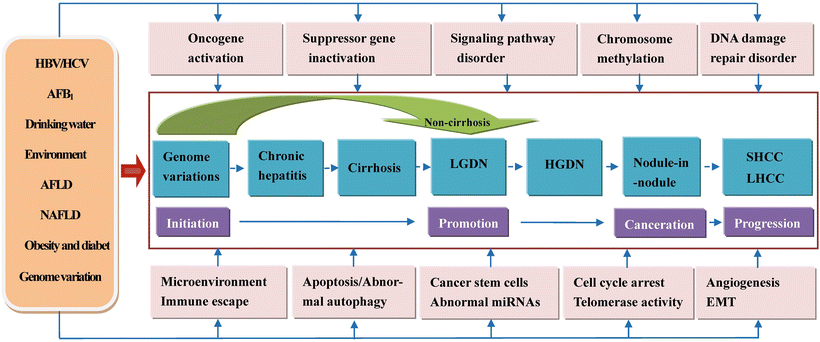

Fig. 7.3
Molecular mechanism of multistage carcinogenesis and progression of hepatocellular carcinoma
7.1.1.4 Clinical Features
According to the statistics of clinical data in 28,869 cases of surgically excised HCC cases in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, the ratio of male to female was 6.72:1, and the average age of HCC patients in each year during the 30 years was stable at around the age of 50 years old, younger than the average age of ICC patients of 54.9 years old and older than the average age of patients with benign liver tumors of 44.9 years old. And 86% of the HCC patients had a history of HBV infection, while about 10% of the patients had a history of HCV infection. The serum AFP level increased with the growth of the tumor, and the positive rates of serum AFP were 69.6, 59.1, 57.6, and 68.2% in HCC patients with tumors <1 cm, <2 cm, <3 cm, and >3 cm in diameter, respectively, with 20 μg/ml as the threshold, while the positive rates of serum AFP were 39.6, 25.7, 26.4, and 44.7% with 400 μg/ml as the threshold, showing that the serum AFP is still one of the serological markers for diagnosis of HCC, but negative serum AFP was found in more than 50% of these patients with HCC.
Most of HCC patients with tumor <3–5 cm in diameter are in early subclinical stage and may manifest no obvious clinical symptoms or signs, while the manifestations in HCC patients with tumors >3–5 cm in diameter include pain in hepatic zone, hepatosplenomegaly, general gastrointestinal symptoms (abdominal distention, diarrhea, decreased appetite), upper abdominal mass, fatigue, emaciation, persistent fever, jaundice, weight loss, abnormal liver function, and paraneoplastic syndrome.
According to clinical features, HCC can be divided into cirrhosis-type HCC characterized by hepatic cirrhosis, portal hypertension, and upper gastrointestinal hemorrhage; fever-type HCC with fever, increased white blood cells, and multiple concurrent infections, which is similar to liver abscess as the main characteristics; hepatitis-type HCC with progressive liver failure and hepatic encephalopathy (hepatic coma) in severe cases, similar to acute severe hepatitis; acute abdomen-type HCC with tumor rupture hemorrhage as the first symptom; cholestasis-type HCC manifested obstructive jaundice involving common bile duct; and metastasis-type HCC with metastasis of extrahepatic organs as the first clinical manifestation.
Type B ultrasonic (B-US) images display hypoechoic lesions in small HCC and hypoechoes or mixed echoes in large HCC, and the detection rate was 85–95% for lesions of 3–5 cm in diameter, while the sensitivity can reach 60–80% for lesions of 1 cm in diameter. Computer tomography (CT) images are more clear and stable with high resolution and show low densities for HCC lesions. Dynamic enhancement CT shows a curve of rapidly increased and then rapidly decreased densities of contrast agent in arterial phase, showing the characteristic “fast in fast out” performance. Magnetic resonance imaging (MRI) images of HCC is characterized by low signal on T1-weighted images and high signal on T2-weighted images, and MRI is especially sensitive for small lesions.
7.1.1.5 Gross Features
- (1)
Gross classification of HCC
Current gross classification of HCC mainly includes the following three models:
- 1.
Eggel’s classification: Suggested by Eggel (1901), the HCC can be divided into nodular type (<10 cm in diameter), massive type (>10 cm in diameter), and diffuse type (cancer nodules in varying sizes and diffuse distribution throughout the liver).
- 2.
Chinese’s classification: Formulated by Chinese Cooperation Group on Pathology of Liver Cancers in 1979 and included in Standards of Diagnosis and Treatment for Malignant Tumors promulgated by the Ministry of Health, the Chinese’s classification divided HCC into five major types and six subtypes, namely:
- ①
Diffuse type: small nodules in diffuse distribution in the liver.
- ②
- ③
Block type: The tumor is 5–10 cm in diameter and can be divided into solitary (Fig. 7.6), confluent, and multiblock types according to the number and the morphology of the lesions.
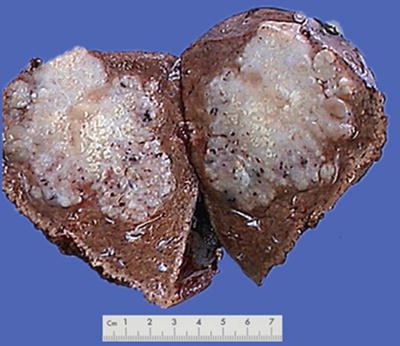
Fig. 7.6
HCC, block type, infiltrative growth without encapsulation
- ④
Nodular type: The tumor is 3–5 cm in diameter and can be divided into solitary (Fig. 7.7), confluent (Fig. 7.8), and multinodular types (Fig. 7.9) according to the number and the morphology of the lesions.
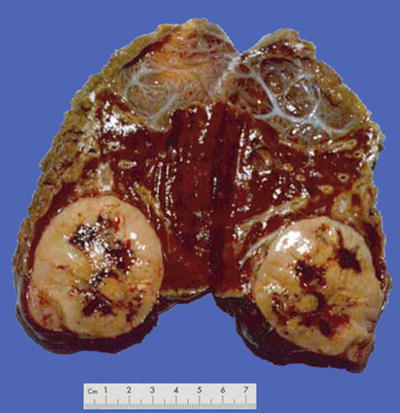
Fig. 7.7
HCC, solitary type, clear boundary with hepatic cyst
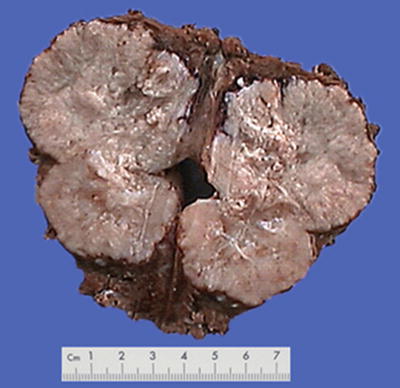
Fig. 7.8
HCC, confluent type, two fused tumor nodules in a gourd shape
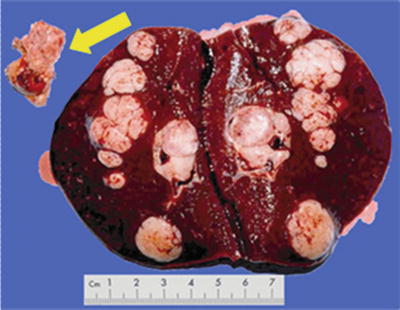
Fig. 7.9
HCC, multinodular type, multiple tumor nodules with portal vein tumor thrombus (arrow)
- ⑤
- ①
According to the statistics on the pathological data of 8580 cases of HCC with surgical resection in our hospital during 2009–2011, huge, massive, nodular, and small types of HCC accounted for 15.1, 30.2, 27.8, and 26.9%, respectively. Following the late 1970s when Chinese Cooperation Group on Pathology of Liver Cancers first proposed the pathological characteristics of small HCC ≤3 cm in diameter and classified it as an independent type, we suggested that ≤3 cm small HCC was a key transition of biological characteristics between benign and malignant tumors in the 1990s (refer to the chapter Small Hepatocellular Carcinoma ). In the Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update issued by the Chinese Pathological Group of Hepatobiliary Tumor and Liver Transplantation, a single tumor ≤1 cm in diameter is defined as minute HCC, and a single tumor with a diameter from >1 cm to ≤3 cm is defined as SHCC (Fig. 7.11). All the above viewpoints represent the basic understanding of gross typing of liver cancers in China at present (Fig. 7.12). In a word, HCC is currently in a lack of a unified international standard on the gross classification, but the major trend is to combine morphological, biological, and molecular characteristics affecting the clinical prognosis, and analysis of gene spectrum may lead to the suggestion of a new model of HCC molecular typing.
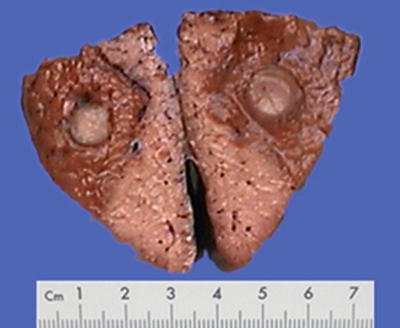
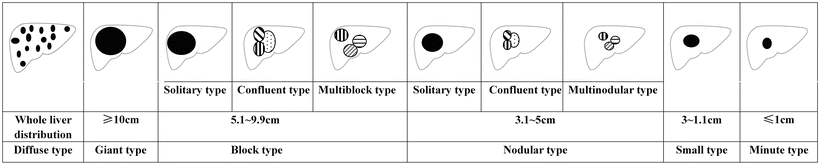

Fig. 7.11
Minute HCC, without cirrhosis

Fig. 7.12
Gross pathological classification of liver cancer in China
- 3.
Kanai’s classification: Nodular HCC was suggested to be divided into three types by Kanai et al. in 1987: type I, solitary nodule type; type II, solitary nodule type with extranodular growth; and type III, contiguous multinodular type [17]. The rate of tumor thrombus and intrahepatic metastasis is the highest in type II (71.4%) and the lowest in type I (7.7%), and the response to TAE is poor, and the prognosis is the worst in the type III.
- 4.
Kojiro’s classification: Proposed by Nakashima and Kojiro in 1987 and based on the gross classification of Okuda in 1984, Kojiro typing classified HCC into five major types and four subtypes:
Infiltrative type (type I): Dissemination in the adjacent liver tissues.
Expansive type (type II): The tumor grows expansively and compresses the surrounding tissue, with a clear border, including single nodular type and multiple nodular type.
Mixed infiltrative and expansive type (type III): Single nodular mixed type and multinodular mixed type.
Diffuse type (type IV).
Special type (type V): such as exophytic HCC.
Exophytic HCC may be connected to the hepatic capsule by fibrous pedicles or directly adheres to the visceral or diaphragmatic surface of the hepatic capsule, and the outward growth of the main part of the tumor into extrahepatic regions can be found due to low resistance, compressing the surrounding organs, while the liver parenchyma is less involved, similar to abdominal massed clinically. One case of HCC in the caudate lobe of the liver was reported in China, with a tumor of 21 cm in diameter protruding into the lessor omental bursa. Among the eight cases of huge exophytic HCC with surgical resection reported by Zhang HB of our hospital, the average diameter of the tumors was 18 cm, and all the patients had a history of HBV infection, of which seven cases concerned lesions on the visceral surface of the liver, seven cases with different degrees of invasion into the adjacent hepatic lobes, and patients in six cases demonstrated long-term survival.
- (2)
Characteristics of gross specimens
Morphological features of HCC include the tumor size, number, and the association with the surrounding liver tissue, such as capsular integrity, focal infiltration, cancer embolus in the vessels, satellite nodules, intrahepatic metastasis, and other biological behaviors, all of which are the main basis for pathological typing of HCC. The section of a HCC mass is often solid, gray white, and soft in texture, with hemorrhage and necrosis, or dark green in cases with cholestasis (Fig. 7.13), dark brown in cases with severe hemorrhage (Fig. 7.14), and pale yellow due to severe fatty degeneration (Fig. 7.15). Cystic degeneration can be observed in cases with severe liquefaction necrosis, and fibrous scars can be found in the lesions of sclerosing HCC. Special attention should be paid to the invasion of capsule and the boundary invasion (Fig. 7.16).
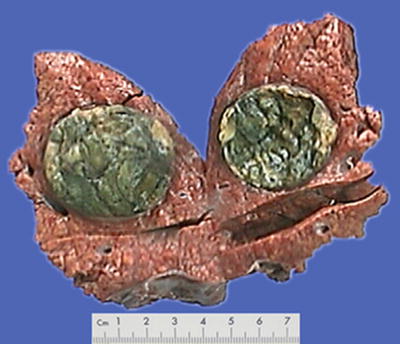
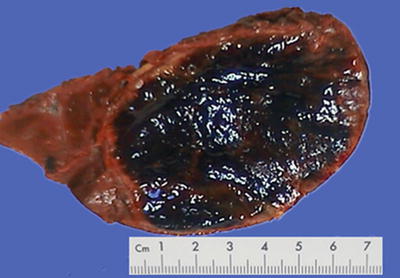
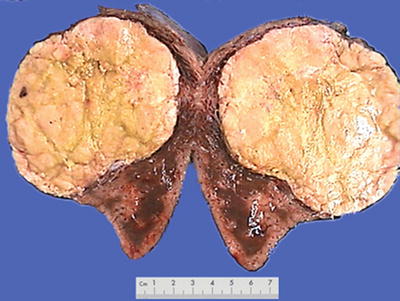
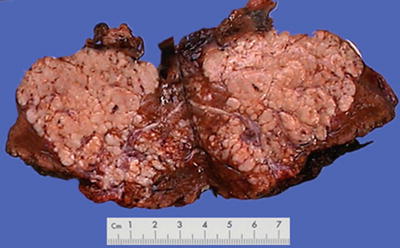

Fig. 7.13
HCC, block type, green-colored tumor by bile staining

Fig. 7.14
HCC, block type, dark brown by severe hemorrhage

Fig. 7.15
HCC, block type, pale yellow by severe steatosis

Fig. 7.16
HCC, block type, with a multifocal growth pattern
In addition, cirrhosis-like HCC (CL-HCC) was also reported in the literature, characterized by diffuse micronodular cirrhosis like microcarcinoma in the background of cirrhosis [18], with mild elevation in serum AFP level and difficulty in distinguishing it from liver cirrhosis nodules. Although it is similar to diffuse HCC, most CL-HCC nodules have a fibrous capsule and a clear boundary. Histologically, it is composed by moderate, well-differentiated HCC cells, often with visible pseudoglandular tubular structures, and 80% of the cases contain visible Mallory bodies. These morphological features of CL-HCC suggest its polyclonal origin, and liver transplantation is a choice for the treatment during which cirrhosis lesions and tumor nodules in the liver can be removed.
- (3)
Sampling of gross specimen
With the deepening understanding on the biological characteristics and tumor microenvironment of HCC, and based on the clinical demand for prognosis evaluation and individualized treatment, more attention should be paid to the examination on invasion of the surrounding liver tissue (microvascular invasion and satellite lesions) and the lesions around the tumors (precancerous lesions ). Therefore, the pathological sampling should focus on comprehensive evaluation of the condition of the tumor and the adjacent liver tissues, rather than tumor itself as previous experience, and the specification of sampling will directly affect the statistical accuracy and scientific significance of pathological parameters (number and distribution of microvascular invasion and satellite foci). According to our experience in operability of practical work in the Department of Pathology, the basic method of sampling for HCC is as follows. Make vertical sections in an interval of 0.5–1 cm, select a representative one, and harvest 7-point baseline sampling protocol, which stipulates that at least four tissue specimens should be sampled at the junction of the tumor and adjacent liver tissues (1:1 ratio) at the 12, 3, 6, and 9 o’clock reference positions, making sure that every sample contains both the tumor tissue and the peritumorous liver tissue for observation of the invasion of capsule, blood vessels, and the surrounding liver tissue. For the purposes of molecular pathological examination, at least one specimen should be sampled at the intratumoral zone. In addition, harvest of liver tissue within the distance of ≤1 cm (adjacent peritumoral liver tissues) and >1 cm (distant peritumoral liver tissues) (Fig. 7.17) is for observation of satellite foci, microvascular invasion , residual cancer cells, as well as the background of the liver tissue (inflammation, fibrosis, cirrhosis). Sampling in the cutting edge should be used to determine whether a positive cutting edge can be found.
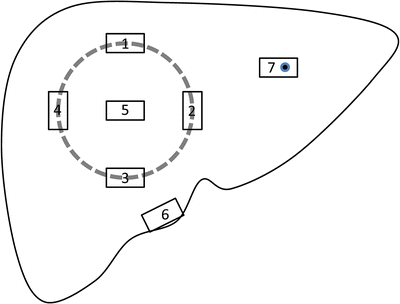

Fig. 7.17
Illustration of sampling HCC specimen
Of course, the number and sites of the samples depend on the size, shape, and number of the lesions of the tumor. For ≤3 cm small HCC, the whole tumor with peritumorous tissue should harvested, and more samples should be harvested corresponding the increased amount of peritumorous liver tissue and number of tumor nodules. The size of each sample should be 1.5–2.0 cm × 1.0 cm × 0.2 cm, and the sampling sites should be marked, with picture of the samples taken for file keeping.
7.1.1.6 Microscopic Features
- (1)
Histological classification
The architectural patterns of HCC mainly include the following types:
- 1.
Thin trabecular pattern: This is a common histological type of well-differentiated HCC. The cancer cells are arranged in 1~3 layers of cell-thick cords between which are micro-blood vessels lined with endothelial cells, similar to normal hepatic cords (Fig. 7.18), and should be carefully distinguished especially in cases with no capsule around the tumor and transition is found between it with the surrounding trabeculae hepaticae (Fig. 7.19). When a fibrous capsule is visible, increased blood sinus gaps are found between hepatic cellular cords, and well-differentiated HCC is located on the side with disorderly arrangement (Fig. 7.20). A diffuse-type distribution of capillarization (microvessel structure) shown in CD34 staining is helpful for diagnosis (Fig. 7.21). And high-differentiated HCC should be differentiated from focal nodular hyperplasia of the liver, hepatocellular adenoma , and high-grade dysplastic nodules .
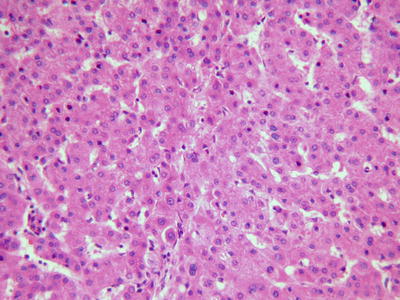
Fig. 7.18
HCC, thin trabecular pattern in 1–3 cells thick
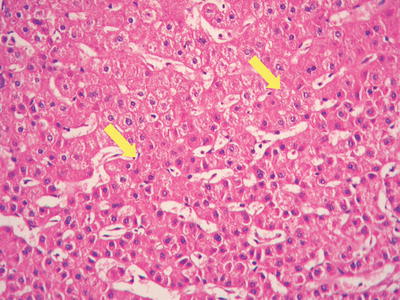
Fig. 7.19
HCC, transition between HCC thin trabecular plates and liver cell cords
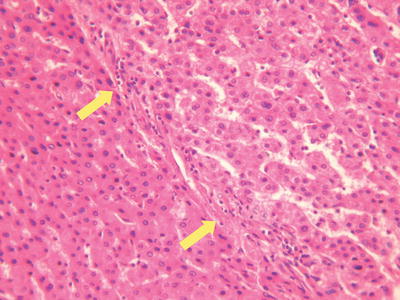
Fig. 7.20
HCC, thin fibrous capsule around tumor tissue
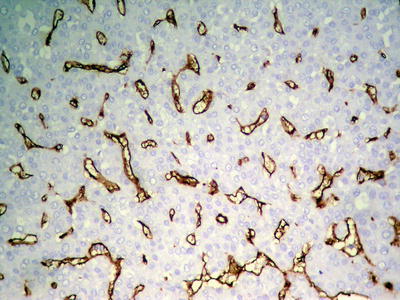
Fig. 7.21
HCC, thin trabecular pattern, CD34 immunohistochemical staining showing diffuse and uniform distribution of microvessels
- 2.
Thick trabecular pattern: This is the most common histological type of moderately differentiated HCC. The cancer cells are arranged in a thick trabecular pattern with four to several layers of cells which have increased nuclear to cytoplasmic ratio, obvious nuclear atypia, and increased mitotic nuclear divisions (Figs. 7.22 and 7.23), and CD34 staining shows the thick cellular cords outlining the sinusoid-like blood spaces (Fig. 7.24).
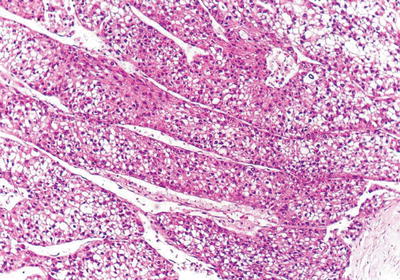
Fig. 7.22
HCC, thick trabecular pattern, HCC cells arranged as thick trabecular cords
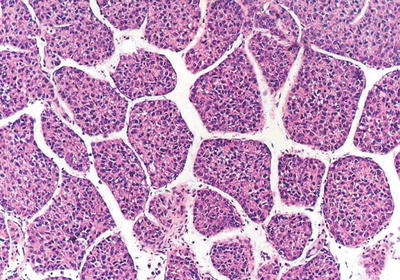
Fig. 7.23
HCC, thick trabecular pattern, ten cells or more thick
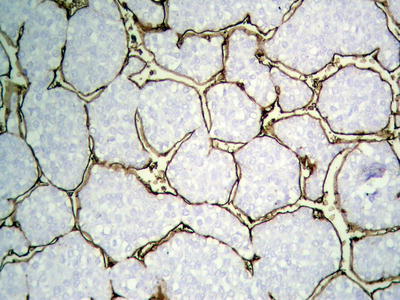
Fig. 7.24
HCC, CD34 immunohistochemical staining outlining thick trabecular cords
- 3.
Pseudoglandular pattern: It is also known as acinar type, which was considered to be formed by expansion of bile canalicular-like structures between the cancer cells, and the glandular tubes are lined with a single layer of cuboidal epithelioid HCC cells (Fig. 7.25), often containing light-stained proteinaceous material in the dilated lumen with absorptive vesicles in the surroundings, similar to thyroid follicle-like structures (Fig. 7.26). The acini may also contain bile (Fig. 7.27). And the paratumorous new foci can also be well-differentiated pseudoglandular-type HCC (Fig. 7.28).
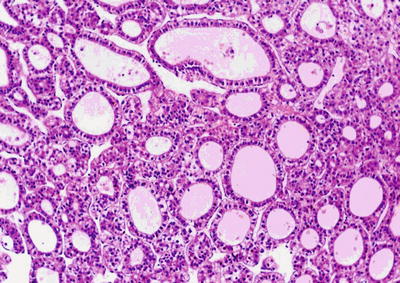
Fig. 7.25
HCC, pseudoglandular pattern, small cuboidal epithelioid HCC cells arranged in gland-like/tubular structures
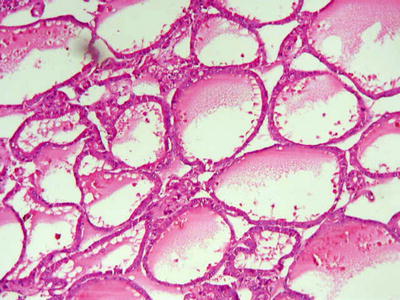
Fig. 7.26
HCC, pseudoglandular pattern, pseudoglands with cystic dilatation containing proteinaceous, similar to the thyroid follicles
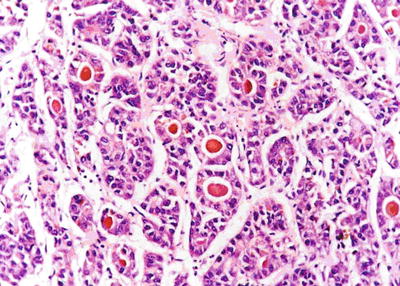
Fig. 7.27
HCC, pseudoglandular pattern with bile plugs
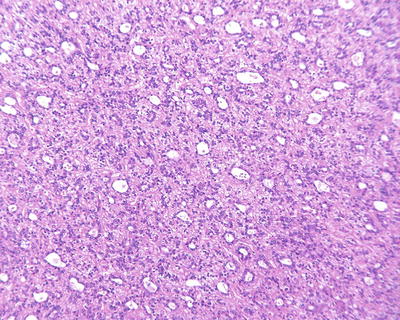
Fig. 7.28
HCC, pseudoglandular pattern, showing diffuse pseudoglandular structures
Cases with diffuse pseudoglandular structures should be distinguished from intrahepatic cholangiocarcinoma and metastatic adenocarcinoma in the liver. These pseodoglandular tubes are positive for a hepatocellular marker Hep Par-1 (Fig. 7.29), and polyclonal carcinoembryonic antigen (CEA) and CD10 staining shows a characteristic canalicular staining pattern on the membrane of the pseudoglandular cells (Fig. 7.30), which suggest these are liver cells rather than real glandular epithelium. CK19 staining demonstrates generally negative results, and its positive results may suggest bile duct epithelial differentiation of the HCC cells (Fig. 7.31), and it belongs to a new subtype of HCC, so we named dual-phenotype HCC (see below). Pseudoglands do not contain myxoid components, and AB/PAS mucus staining is negative, and MUC-1 staining shows bile-like secretion (Fig. 7.32), also suggesting the lumen derives from specialized transformation of capillary bile ducts.
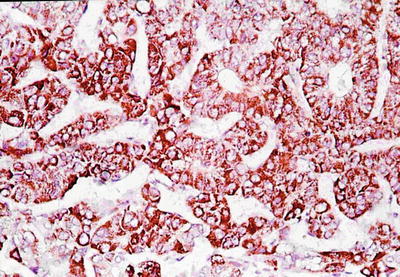
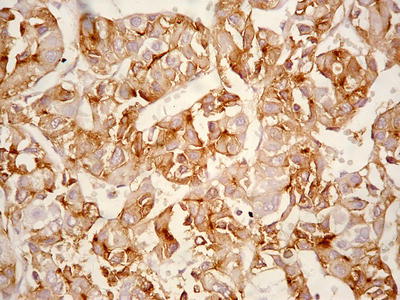
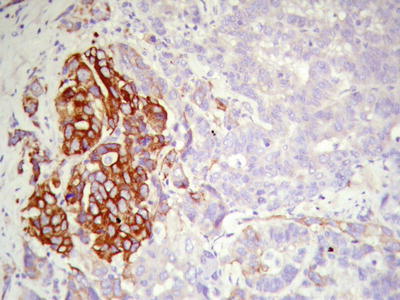
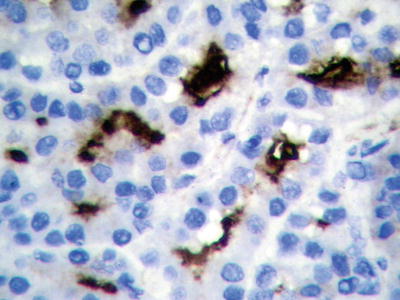

Fig. 7.29
HCC, pseudoglandular pattern, showing positive immunohistochemical staining of Hep Par-1

Fig. 7.30
HCC, pseudoglandular pattern, immunohistochemical staining ofCD10 showing canalicular membrane of HCC cells

Fig. 7.31
HCC, pseudoglandular pattern, immunohistochemical staining showing some CK19-positive HCC cells

Fig. 7.32
HCC, pseudoglandular pattern, immunohistochemical staining showing MUC-1-positive glandular tubules
- 4.
Compact pattern: It is also known as solid type. The cancer cells are arranged in flaky or solid patterns, with insignificant or slit-like hepatic sinusoid capillarization due to serious compression (Figs. 7.33, 7.34, and 7.35), suggesting active growth and poor differentiation of the tumor cells. And CD34 immunohistochemical staining shows sparsely distributed microvessels, significantly different from densely arranged microvessels of trabecular-type HCC (Fig. 7.36).
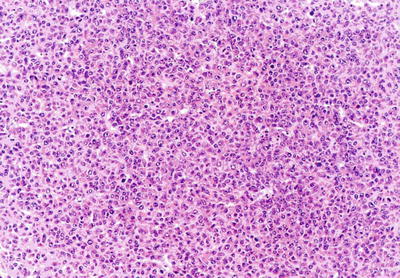
Fig. 7.33
HCC, compact pattern, closely arranged cells with no obvious hepatic sinusoids
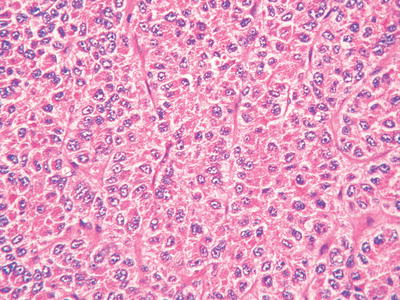
Fig. 7.34
HCC, compact pattern, HCC cells arranged in solid cord-like structures with no obvious hepatic sinusoids
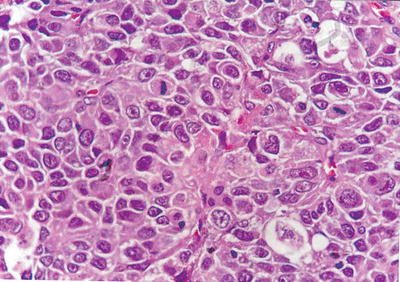
Fig. 7.35
HCC, compact pattern, showing a mosaic arrangement of tumor cells with frequent mitotic figures
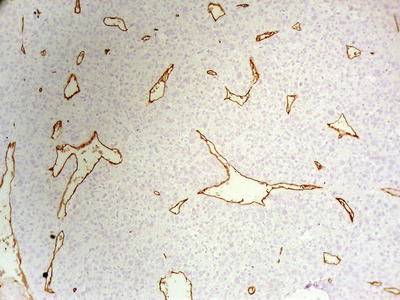
Fig. 7.36
HCC, compact pattern, CD34 staining showing sparse microvessels
- 5.
Sclerosing (or scirrhous) pattern: Visible gray-white fibrous scars can be found on the section of the tumor (Figs. 7.37 and 7.38). Under the microscope, the tumor contains abundant collagen fibrous tissue and is divided by thick fibrous connective tissue into varying sizes of cell nests (Fig. 7.39), similar to the morphology of metastatic tumors or intrahepatic cholangiocarcinoma in some cases (Fig. 7.40). And hyaline degeneration can also be observed in some cancer cells. Positive Hep Par-1 staining (Fig. 7.41) is helpful in differential diagnosis. Sclerosing type of HCC indicates a strong local immune response in the body, which is also common as a histological reaction in cases treated with radiotherapy or chemotherapy.
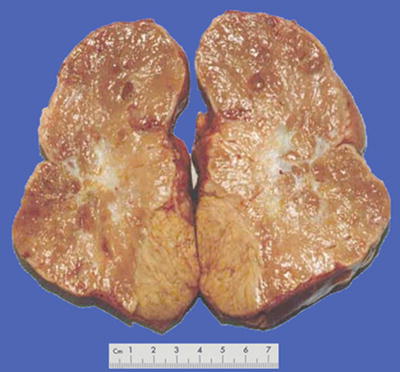
Fig. 7.37
HCC, sclerosing type, showing central fibrous scar
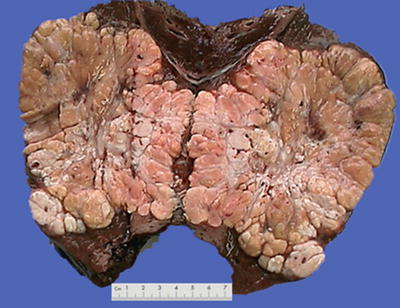
Fig. 7.38
HCC, sclerosing type, showing lobulated mass due to fibrous septa
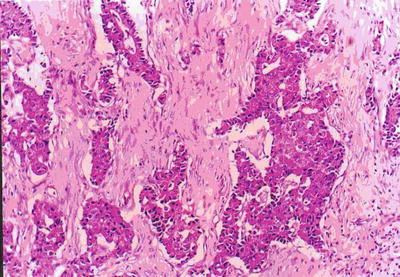
Fig. 7.39
HCC, sclerosing type, showing nested structures divided by fibrous septa
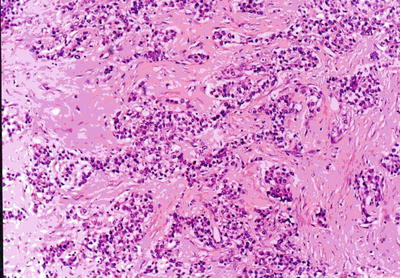
Fig. 7.40
HCC, sclerosing type, tumor cells arranged in solid nests with rich fibrous stroma
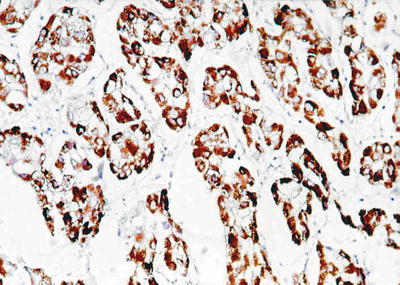
Fig. 7.41
HCC, sclerosing type, showing positive immunohistochemical staining of Hep Par-1
- 6.
Purpura pattern: The tumor is rich in highly dilated vessels containing abundant blood in the lumen, and the sections are often dark red, similar to that of the hemangioma (Fig. 7.42). Under a microscope, blood sinuses in the tumor tissue are highly expanded or similar to cavernous hemangioma -like structures (Figs. 7.43 and 7.44), with flat peripheral cancer cells due to compression. In addition, focal purpura-like vascular expansion can be observed in many HCC tissues.
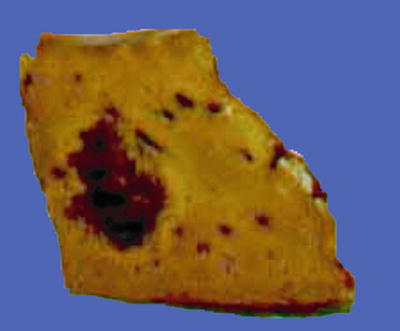
Fig. 7.42
HCC, purpura pattern, the cut surface looks like hemangioma
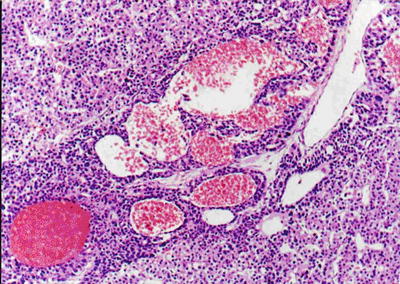
Fig. 7.43
HCC, purpura pattern, the tumor tissue contains dilated lacunae vasorum
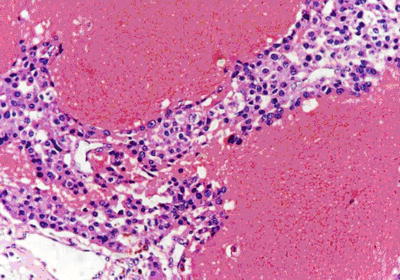
Fig. 7.44
HCC, purpura pattern, the dilated lacunae vasorum contains numerous erythrocytes
- 7.
Rosette-like pattern: Little round groupings of HCC cells consist of a spoke-wheel or halo arrangement surrounding a central, acellular region. A minority of HCC tissue can be arranged in a rosette-like structure, with each rosettes surrounded by about more than 20 cells at an equal interval distance in peripheral regions of the rosettes, with or without significant central lumen. The cells are consistent in size with eosinophilic cytoplasm and unclear boundaries (Fig. 7.45a, b), and immunohistochemistry shows strongly positive Hep Par-1 (Fig. 7.46).
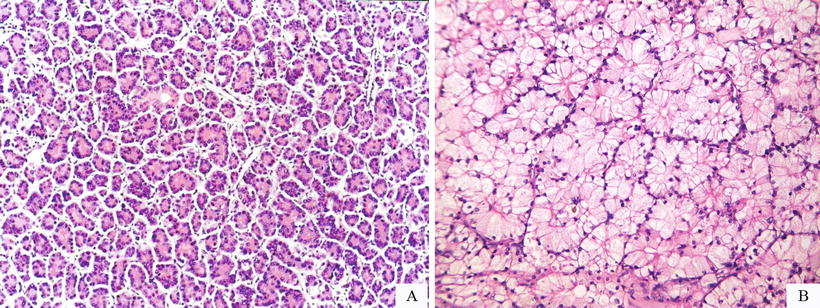
Fig. 7.45
(a) HCC, rosette-like pattern, tumor cells surrounding a central lumen that contains cytoplasmic extensions from the tumor cells, (b) HCC, rosette-like pattern, concentric arrangement of rosette-like hepatocytes with bright cytoplasm
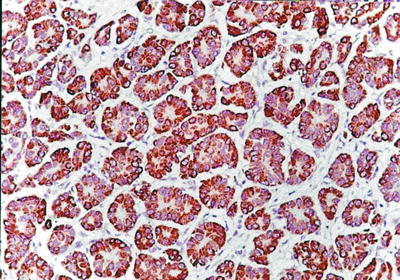
Fig. 7.46
HCC, rosette-like pattern, showing positive immunohistochemical staining of Hep Par-1
- 8.
Private-like arrangement HCC cells contain sparse cytoplasm and vacuolation, with small remaining of pale-stained cytoplasm. The blue-stained nuclei of the cancer cells are arranged in a single layer near the sinusoidal surface of the trabecular structure which is like privates (Fig. 7.47), and CD34 staining shows increase of microvascular density (Fig. 7.48).
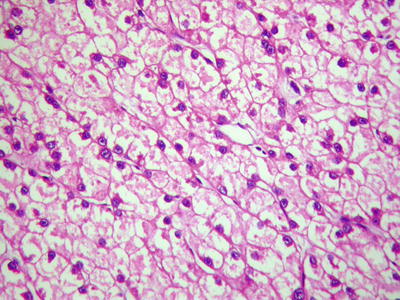
Fig. 7.47
HCC, private-like arrangement, cancer cells are arranged in a single layer along the trabecular cords
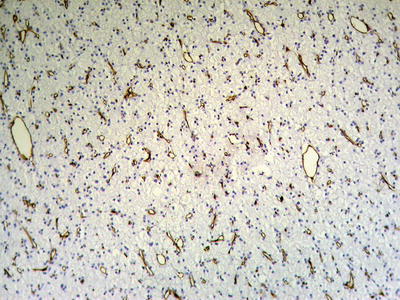
Fig. 7.48
HCC, private-like arrangement, CD34 staining showing diffuse microvessels
- 9.
Spontaneous necrosis type: The diagnosis of HCC is based on the clinical history of viral hepatitis in patients, previous elevated serum AFP level, liver masses found in imaging examination, as well as other clinical indications. And in cases without any special treatment, after the levels of serum AFP decreased or become negative, complete coagulation necrosis is found in the tumors. In addition, about 100 cases of spontaneous regression of HCC have been reported in the literature, and the imaging results during the follow-up for HCC patients showed part or complete disappearance of liver masses or extrahepatic metastases. And I diagnosed one case in accordance to the criteria of spontaneous necrosis-type HCC, and it was treated with surgical resection, the lesion of which demonstrated severe hemorrhagic necrosis in gross appearance (Fig. 7.49), while repetitive sampling failed to find remaining cancer cells, but only the remaining trabecular contour left by tumor necrosis (Fig. 7.50). The patient recovered well with a good prognosis and no recurrence was found during the long-term postoperative follow-up. And theses HCC cases with spontaneous necrosis or spontaneous regression are likely to be related to the strong immune function of the patients.
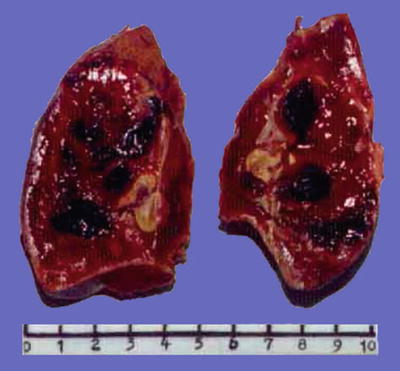
Fig. 7.49
HCC, spontaneous necrosis type, showing severe hemorrhage and necrosis
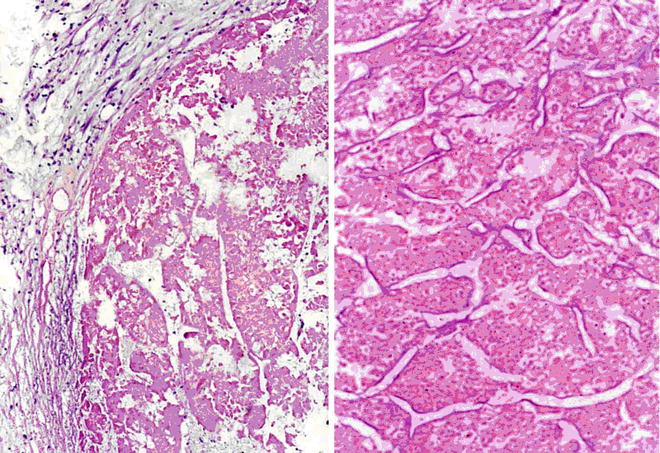
Fig. 7.50
HCC, spontaneous necrosis type, tumor tissue showed a complete coagulation necrosis, only remaining trabecular outline
Supplementary: Posttreatment Necrotic HCC
Minimally invasive surgical methods are widely applied in the treatment of HCC, including transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), microwave coagulation therapy (MCT), laser thermal ablation (LTA), and argon helium cryoablation surgery (AHCS), which can directly cause the coagulation necrosis of HCC cells and tissues. The main mechanism of minimally invasive treatment for hepatic tumors is physical or chemical destruction or ablation of cancer cells, so as to achieve the purpose of effective decrease of tumor load. However, the biggest difference between it and surgical resection is the tumor remaining in situ after interventional therapy; therefore, the efficacy of destruction or elimination of tumor cells is closely related to the effect of the interventional therapy which is affected by various important factors, such as tumor size, number, location, shape, growth pattern, biological characteristics, etc. Thus, in pathological examination, sampling in multiple parts and sites should be conducted as well as careful searching for tumor cells. The degree and scope of necrosis in the cancer tissue should also be described in pathological reports. Single-stranded DNA markers, lactate dehydrogenase staining, and Ki-67 labeling are all methods to determine the activity of degenerated and necrotic cancer cells. According to incomplete statistics, 102 cases of HCC treated by interventional therapy and subsequent surgical resection have been found in our hospital from September 2005 to November 2007, and pathological examinations displayed 78 cases (76.5%) of complete necrosis and cancer cell remaining in 24 cases (23.5%) (Figs. 7.51, 7.52, 7.53, and 7.54).
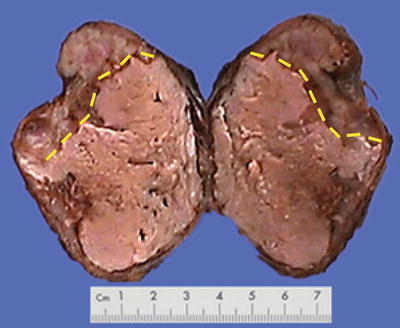
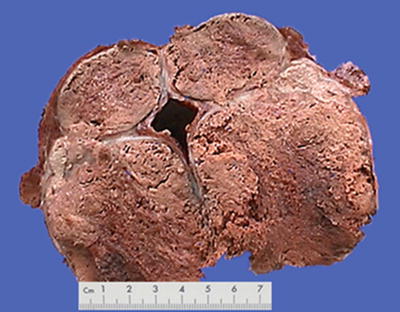
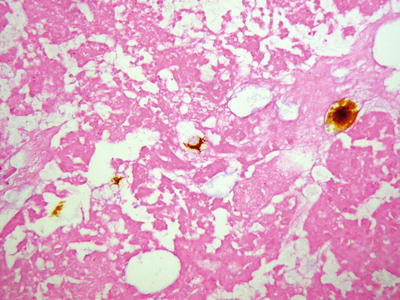

Fig. 7.51
Necrosis of HCC after TACE

Fig. 7.52
Necrosis of HCC after TACE

Fig. 7.53
Necrosis of HCC after TACE, no survival cancer cells under microscope

Fig. 7.54
Necrosis of HCC after TACE, a small amount of incompletely necrotic cancer cells under microscope
- (2)
Cytological classification of HCC
HCC cells have a variety of morphological manifestations, even completely different from that of hepatocytes, mainly including the following types:
- 1.
Liver cell type: This is the most common type, similar to that of normal hepatocytes, and the cancer cells were polygonal, with eosinophilic fine granular cytoplasm. The cell membrane contains specialized bile canalicular structure and bile plug, which is an important sign of hepatocyte differentiation. Poorly differentiated cancer cells are enlarged in volume significantly, with increased cytoplasmic basophilia, nuclear volume, and nucleus to cytoplasm ratio, and the nuclei are irregular shaped, darker stained with a variable number of mitotic figures.
- 2.
Clear cell type: More than 50% of the cancer cells contain rich glycogens which are irregular and large vacuole-like structures, resulting in transparent cytoplasm (Fig. 7.55), and nucleus can be found floating in the center of the cytoplasm (Fig. 7.56). The cancer cells are positive in PAS staining because of abundant glycogen content. In cases with transparent cells, as the majority of the tumor, it should be differentiated from metastatic clear cell carcinoma which originates in the kidney, while the latter is positive for EMA, Leu M-1, and broad-spectrum CK staining but negative for Hep Par-1, which is positively expressed in clear cell-type HCC (Fig. 7.57). Emile et al. (2001) studied tumor diameter, tumor number, and prognosis and conducted the detection of six microsatellite loci of the clear cell-type HCC; however, no obvious difference was found between liver cell-type HCC and clear cell-type HCC.
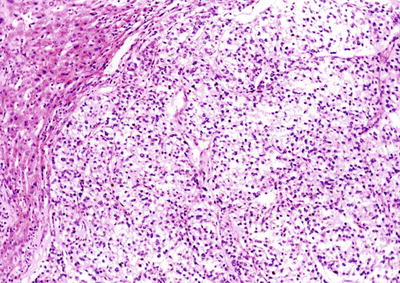
Fig. 7.55
HCC, clear cell type, showing vacuolated cytoplasm in clear cell
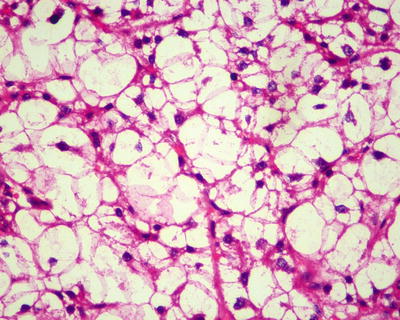
Fig. 7.56
HCC, clear cell type, the tumor cell cytoplasm showed hydropic-type vacuolar degeneration, like empty bubble
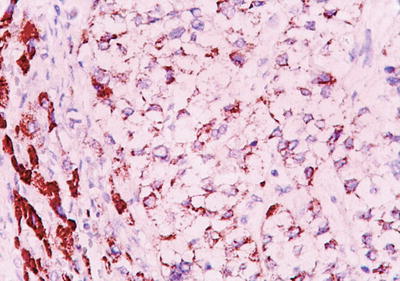
Fig. 7.57
HCC, clear cell type, showing positive immunohistochemical staining of Hep Par-1
- 3.
Fatty-rich type: Also known as fatty change, this type of HCC is formed by cancer cells with metabolism disorder of fat, characterized by circular lipid droplets with smooth surface and consistent size, occupying in the whole cytoplasm (macrovesicular steatosis), leading to nuclear deviation (Figs. 7.58 and 7.59). Occasionally, nucleated red cells can be found in the hepatic sinusoids, suggesting extramedullary hematopoiesis (3–5%). The fatty-rich type of HCC should be differentiated from angioleiomyolipoma and focal fatty change, and typical HCC samples should be harvested via multiple sampling to avoid misdiagnosis as benign lesions. Immunohistochemical staining shows positive results for GPC-3, Hep Par-1 (Fig. 7.60), CD34 (Fig. 7.61), and CK18 to facilitate the diagnosis.
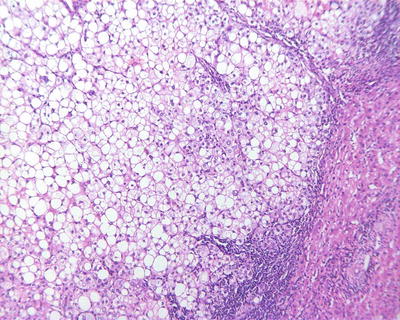
Fig. 7.58
HCC, fatty-rich type, showing diffuse fatty change in HCC cells
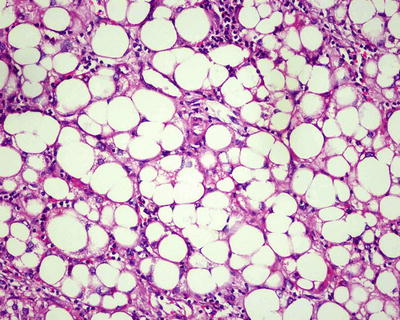
Fig. 7.59
HCC, fatty-rich type, showing hepatic macrovesicular steatosis
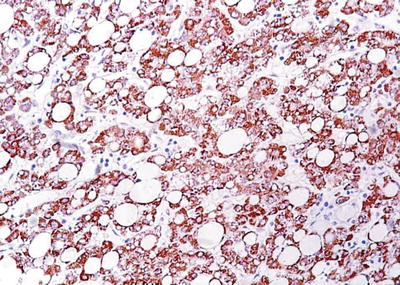
Fig. 7.60
HCC, fatty-rich type, showing positive immunohistochemical staining of Hep Par-1
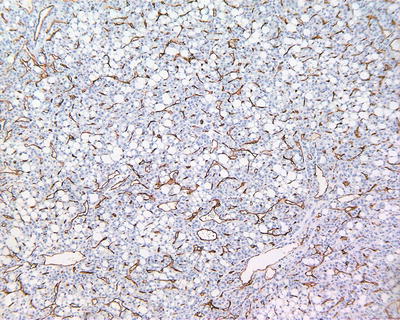
Fig. 7.61
HCC, fatty-rich type, CD34 staining showing diffuse microvessels
- 4.
Spindle cell type: Also known as sarcomatoid type, it accounts for about 5% of HCC and is a special form of poorly differentiated HCC, of which approximately 46% of the patients are found with positive serum AFP. The tumor cells are spindle like with rod-shaped nucleus (Fig. 7.62) in fascicular or storiform arrangement (Figs. 7.63 and 7.64), similar to myogenic sarcoma, fibrosarcoma , or chondrosarcoma, with invasive growth on the boundary of the tumor, and are often concurrent with typical HCC; therefore, adequate samples should be harvested. Immunohistochemical staining shows that spindle cells express Hep Par-1 (Fig. 7.65), AFP, CK, EMA (Fig. 7.66), vimentin (Fig. 7.67), and S-100. Electron microscope demonstrates cancer cells with abundant rough endoplasmic reticulum, phagolysosomes, lipid droplets, and microvillar projections, indicating that spindle cells derive from metaplasia or sarcomatoid change of HCC rather than real mesenchymal components. It can be diagnosed as sarcomatoid carcinoma but should be distinguished from carcinosarcoma , which is composed by both carcinoma and sarcoma elements. Spindle cell-type HCC has a poor prognosis with frequent invasion of portal vein and intrahepatic metastasis.
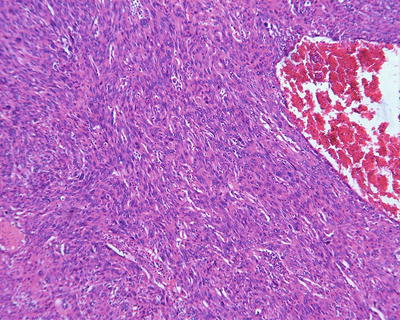
Fig. 7.62
HCC, spindle cell type, the tumors consisted of uniform long spindle cells
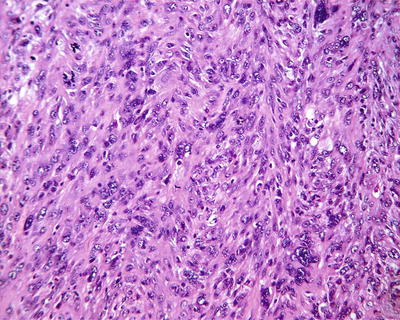
Fig. 7.63
HCC, spindle cell type, spindle tumor cells arranged in an interwoven pattern
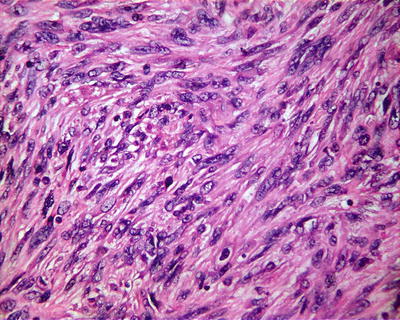
Fig. 7.64
HCC, spindle cell type, showing spindle tumor cells with rod-shaped nucleus
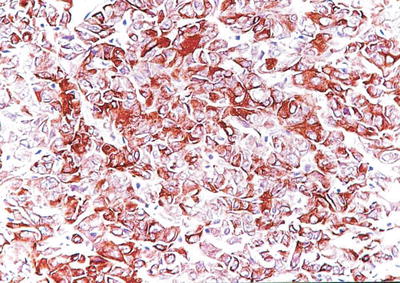
Fig. 7.65
HCC, spindle cell type, showing positive immunohistochemical staining of Hep Par-1
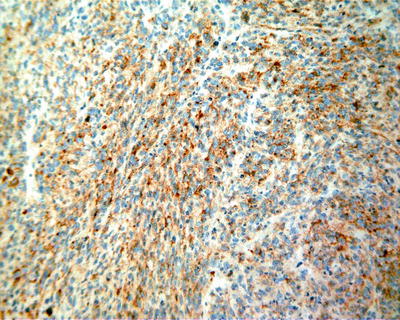
Fig. 7.66
HCC, spindle cell type, showing positive immunohistochemical staining of EMA
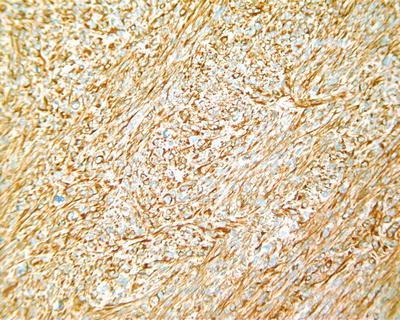
Fig. 7.67
HCC, spindle cell type, showing positive immunohistochemical staining of vimentin
- 5.
Foam cell-like type: According to our observations, the cells of this rare type of HCC are similar to the xanthoma cells, with cell volume one to two times greater than that of normal liver cells. Their cytoplasms are sparse and mesh filamentous, filled with microvesicles, and may also contain tiny fat vacuoles. The nuclei are relatively small in size with no deviation (Figs. 7.68 and 7.69). The cancer cells lose the morphology of a liver cell, but the immunohistochemical staining shows positive Hep Par-1 (Fig. 7.70), which may be due to highly hydropic degeneration of the mitochondria in cancer cells, resulted in a more swelling and sparse cytoplasm than that in clear cell-type HCC.
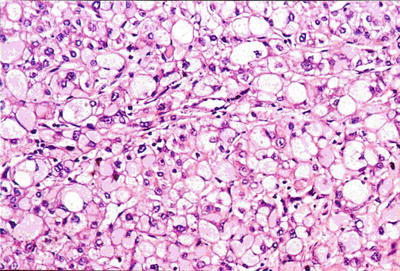
Fig. 7.68
HCC, foam cell-like HCC cells with a swollen vacuolated cytoplasm
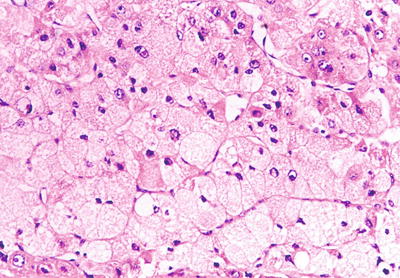
Fig. 7.69
HCC, foam cell-like type, the volume of foam cell-like HCC cells increased obviously
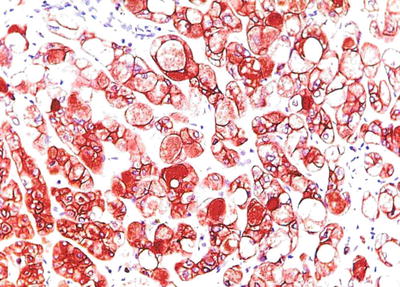
Fig. 7.70
HCC, foam cell-like type, showing positive immunohistochemical staining of Hep Par-1
- 6.
Giant cell type: The cancer cells are pleomorphic in varying sizes and irregular shapes, with a large number of multiple or odd-shaped nuclei with megakaryocytes in horseshoe-shaped arrangement, and nuclear mitotic figures are commonly seen. The cells lack the morphology of liver cells (Fig. 7.71a–d), but immunohistochemical staining shows that they retain the hepatocytic phenotype. In addition, osteoclast-like giant cells can be found in HCC tissues; thus, it is also known as osteoclast-like giant cell tumor of the liver, consisting of small mononuclear cells and osteoclast-like giant cells. The former are positive for AFP and CAM5.2, suggesting the origination from HCC, and it may be a metaplastic change of HCC, and the latter are strongly positive for CD68 and KP1, negative for AFP, CK, and P53, suggesting that it is a kind of reactive histiocyte.
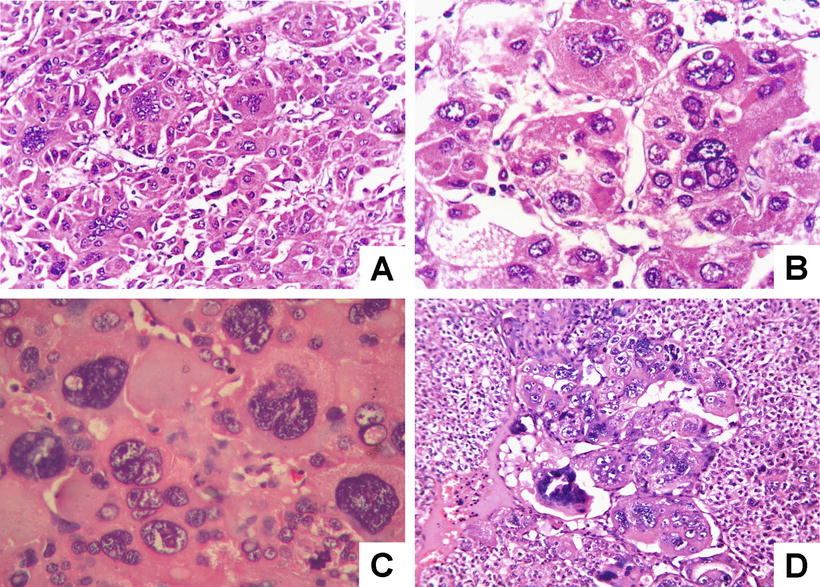
Fig. 7.71
HCC, giant cell type, showing multinucleated giant cells with odd-shaped nuclei
- (3)
Grading of differentiation
Edmondson-Steiner grading is still widely used:
Grade I: The cancer cells are highly differentiated, with no obvious atypia, similar to normal liver cells, which are arranged in thin trabecules, similar to normal hepatic plates.
Grade II: The cancer cells are moderately differentiated with their morphology close to that of normal liver cells, arranged mainly in thin trabecules, but the karyoplasmic ratio is slightly increased, the nuclear staining darker, and the cytoplasm acidophilia increased. On the basis of trabecular structures, pseudoglandular structure may also be found.
Grade III: The differentiation of the cancer cells was poor, and the changes of nuclear volume, atypia, and mitotic figures were more obvious than that in Grade II; occasionally, a few tumor giant cells can be observed.
Grade IV: The cancer cells were undifferentiated or anaplastic, with extremely irregular shapes, or the tumor is an undifferentiated carcinoma. Tumor giant cells or tumor cells with odd-shaped nuclei are commonly seen, and highly pleomorphic carcinoma cells constitute the majority of them, with less cytoplasm, dark-stained nuclear chromatin, loose cell arrangement, and no significant trabecular structure.
In addition, a simple grading method recommended by WHO can also be adopted, with grades including well differentiation, moderate differentiation, poor differentiation, and undifferentiation. A potential relationship has been shown between clinical prognosis and differentiation of HCC; the grading of HCC can provide reference for the evaluation on the biological characteristics of HCC.
- (4)
Growth and infiltration
The diversification of HCC growth and invasion directly reflects the diversification of HCC biology behavior characteristics and is closely related to the prognosis of the patients, as well as an important reference for the designing of clinical individualized treatment mode. To sum up, HCC has at least the following eight patterns of growth and invasion:
- 1.
Capsular invasion: It mainly contains two types. One is invasion inside the capsule, and the tumor has not invaded the whole layers of the capsule and forms tumor thrombus in the capsule (Fig. 7.72). The other is a breakthrough of the capsule, forming satellite foci or tumor embolus outside the capsule. It is noted that the capsule is an important barrier against the dissemination of HCC, and the formation of a second capsule after the breakthrough of the first layer of the capsule is sometimes observed in HCC tissue (Fig. 7.73), as well as multiple layers of fibrous capsule formation (Fig. 7.74). Complete resection of the tumor can be achieved by expanding the resection area to a certain distance adjacent to the tumor capsule. HCC complicated by liver cirrhosis often has a capsule, while HCC cases without cirrhosis often contain no capsule with a potential of invasive growth into paracancerous liver tissue to form multifocal lesions due to lack of fibrous blockage.
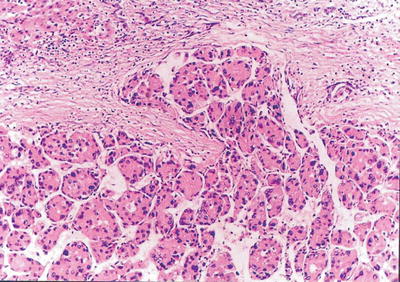
Fig. 7.72
HCC, tumor capsule vessel invasion
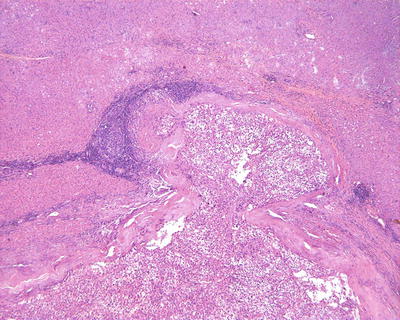
Fig. 7.73
HCC, tumor bursting into the capsule
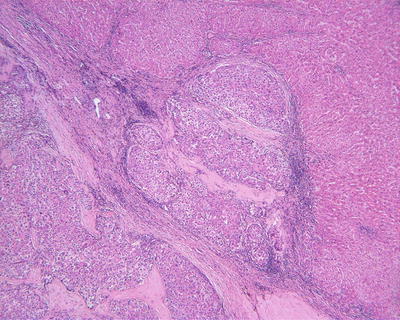
Fig. 7.74
HCC, showing repeated capsule invasion and repeated capsule enveloping
- 2.
Vascular invasion HCC can invade major hepatic vessels with observable tumor thrombus in them both grossly and on images or presents microvascular invasion (MVI) or microvascular thrombus. At present, the definition, diagnostic criteria, and grading system of MVI have not reached a consensus. In view that HCC is rich in sinuses and lacks fibrous interstitial components, many scholars defined MVI as the microscopical observation of tumor thrombus inside the vessels lined with endothelial cells, mainly within the branches of the portal vein and tributaries of the hepatic vein or vessels of the tumor capsule. Occasionally, the liver cancer may invade the hepatic artery, bile duct, and lymphatic vessels, which should be reported independently because of their differences in clinical significance. As for trabecular patterns of HCC lined with sinusoidal endothelial cells, it is not a real MVI (Fig. 7.75). The MVI tends to adhere on endothelial cells or invades the vascular wall, resulting in interruption of the vascular endothelium (Figs. 7.76, 7.77, and 7.78). Studies have shown that the cancer cells must enter the vessels lined by endothelial cells and they can escape from the host’s immune attack and the coagulation cascade to survive and metastate. Other studies showed that >50 suspended carcinoma cells in the portal vein were markedly correlated to the prognosis (Fig. 7.79) [19]. The incidence of MVI increase with the increase of tumor size, which is a key pathological factor leading to high risk of recurrence and poor prognosis of HCC patients after surgical resection.
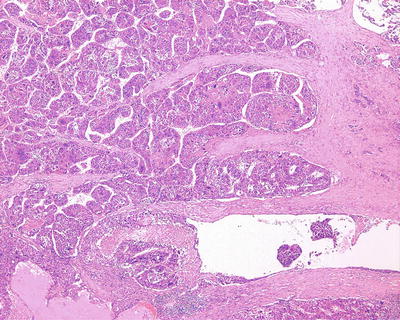
Fig. 7.75
HCC, tumor thrombus formation in tumor stroma
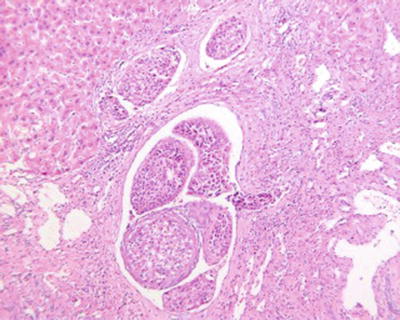
Fig. 7.76
HCC, tumor thrombus in the multilevel branch of the portal vein
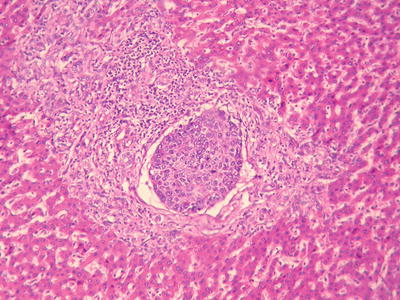
Fig. 7.77
HCC, microvascular thrombus in the portal interlobar vein
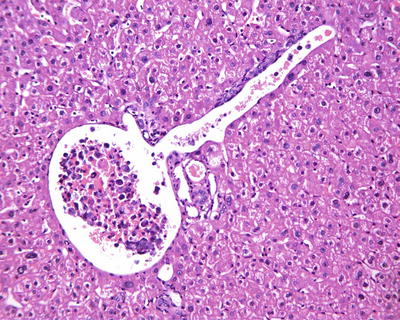
Fig. 7.78
HCC, showing intravascular floating tumor clusters in the branch of portal vein
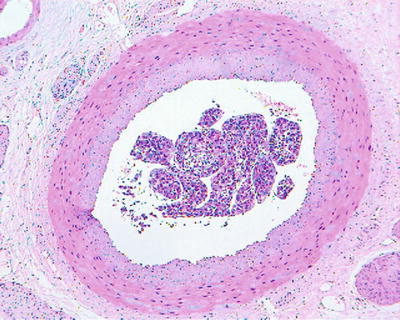
Fig. 7.79
HCC, tumor thrombi involving the branch of the hepatic artery
According to the literature, the incidence of MVI in HCC was 15–57%, which increases with the increase of HCC volume. MVI in the adjacent paracancerous liver tissue can be excised along with the primary tumor in operation, while those located in distant paracancerous liver tissue cannot be removed in whole easily. MVI occurs not only as late stage of HCC, and numerous and distant MVI can increase the risk of recurrence and metastasis of HCC. Most of the studies demonstrate that MVI is an indicator for highly invasive growth of HCC and is one of the independent pathological factors affecting the postoperative recurrence and long-term therapeutic efficacy of HCC, as well as the important pathological evidence for anti-recurrence treatment. Therefore, pathological examinations should include careful observation on the number, distance, vascular types, and distribution of MVI . In addition, lymphatic vessel invasion, metastasis along lymphatic vessels, or tumor thrombi in intrahepatic bile ducts can be found in a few cases of HCC (Fig. 7.80). According to the Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update of China, risk grading of MVI should be done according to the quantity and distribution of MVI : M0, no visible MVI ; M1 (low-risk group), ≤5 MVI in adjacent paracancerous liver tissue (≤1 cm); and M2 (high-risk group), > 5 MVI or MVI in distant paracencerous liver tissue (> 1 cm).
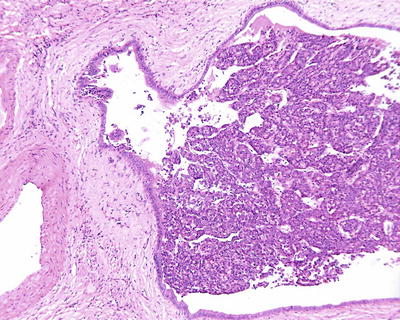

Fig. 7.80
HCC, tumor thrombi involving the branch of bile ducts
To avoid the misdiagnosis of fibrous tissue around the cancer cell nests as MVI , a selection of immunohistochemical staining is used. VEGF, CD31, CD34, SMA (Fig. 7.81), and h-caldesmon can label blood vascular endothelial cells and D2–40, podoplanin, and LYVE-1 can label lymphatic endothelial cells. Rodríguez-Perálvarez et al. (2013) pointed out that tumor thrombus within the vessels wrapped by a smooth muscle can be accurately diagnosed as MVI ; however, micro- veins have only a thin layer of the outer membrane that contains longitudinally arranged collagen fibers and elastic fibers; thus, staining methods for elastic fibers can be applied for identification, including Victoria blue, orcein, and Elastica van Gieson staining (EVG).
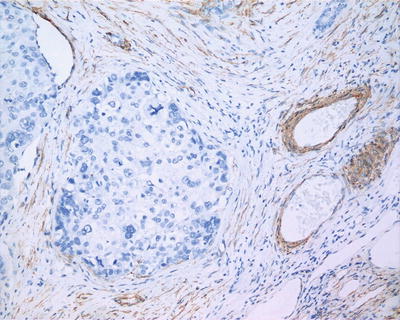

Fig. 7.81
HCC, showing negative immunohistochemical staining of SMA for carcinoma nests or satellites
- 3.
Satellite nodules: Satellite nodules usually refers to small tumor foci located in the paracancerous tissue within a distance of ≤2 cm from the primary tumor, and there is no continuity between them (Fig. 7.82). Those lesions adjacent to the main tumor or the capsule of the tumor are often sub-tumor foci. Lesions located in the paracancerous tissue at a distance >2 cm from the primary tumor (Fig. 7.83), particularly cancer nodules with a diameter >2 cm, can be either intrahepatic metastasis of the primary tumor or new tumor foci, which are usually indistinguishable morphologically, and molecular clone detection should be considered to determine the clonal origin and the nature of these lesions.
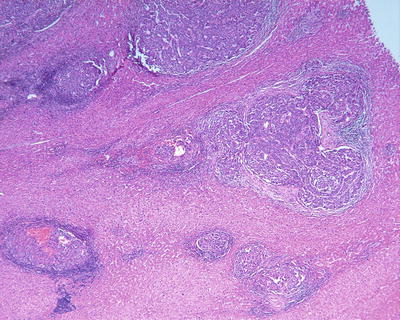
Fig. 7.82
HCC, showing multiple satellite nodules in the peritumoral tissue
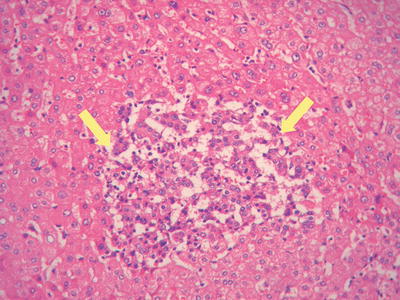
Fig. 7.83
HCC, showing early carcinoma foci inthe peritumoral tissue
- 4.
Early hepatocarcinogenesis: Early hepatocarcinogenesis in the precancerous liver tissues are often well-differentiated foci based on the background of cirrhosis and high-grade dysplastic nodules , exhibiting natural transition between carcinoma trabecules and hepatic plates (Fig. 7.83), and they are the pathological basis of multicentric origin for HCC development.
- 5.
Transition: No significant border can be found between the carcinoma cells and normal liver cells in the pericarcinoma tissues due to the transition or replacement growth without fibrous capsule. The only slight difference between them is the cytoplasmic staining and trabecular width which can be used carefully for identification (Fig. 7.84).
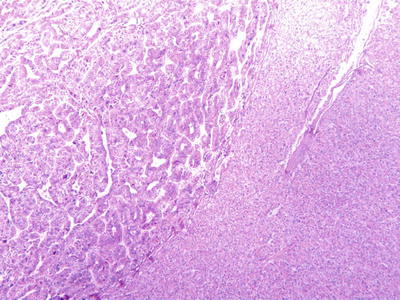
Fig. 7.84
HCC, pseudoglandular pattern with pseudoglandular pattern
- 6.
Multiple histological structures: We observed that about 30% of HCC contain two or more different histological and cytological types, named as HCC with multiple histological structures (MS-HCC) (Figs. 7.85, 7.86, 7.87, 7.88, and 7.89). This phenotype represents one of the HCC heterogeneous features, and it also suggests polyclonal origins of the tumor cells in HCC. We conducted genomic microsatellite variation pattern analysis on the clonal association in MS-HCC tissues and found at least a part of MS-HCC cases have multicentric origins. Theoretically, this multi-phenomenon of MS-HCC may suggest the formation of multiple subclones due to the selection pressure and mutation variations among the carcinoma cells, which may improve the complexity and diversity of biological behaviors as well as the clinical response to the treatment of HCC, and this is worthy of further classification studies [20].
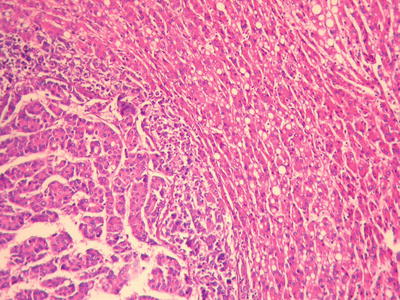
Fig. 7.85
HCC, thin trabecular pattern with thick trabecular pattern
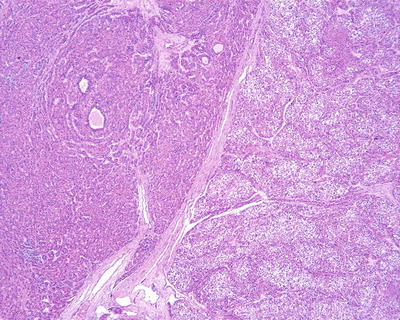
Fig. 7.86
HCC, thick trabecular pattern with thin trabecular pattern
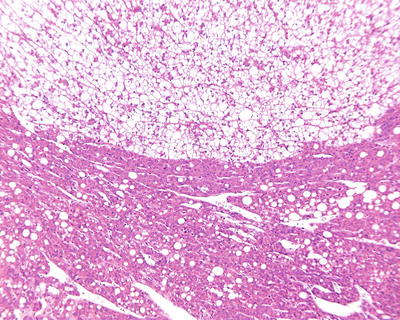
Fig. 7.87
HCC, clear cell type with thin trabecular pattern
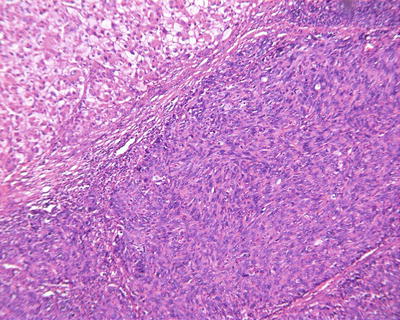
Fig. 7.88
HCC, clear cell type with spindle cell type
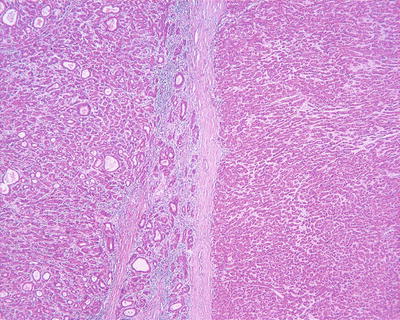
Fig. 7.89
HCC, pseudoglandular pattern with thin trabecular pattern
- 7.
Neural invasion: Invasion of neural tissues by HCC can be found occasionally in a few cases (Fig. 7.90a, b), suggesting that HCC may also be disseminated along the nerve sheath.
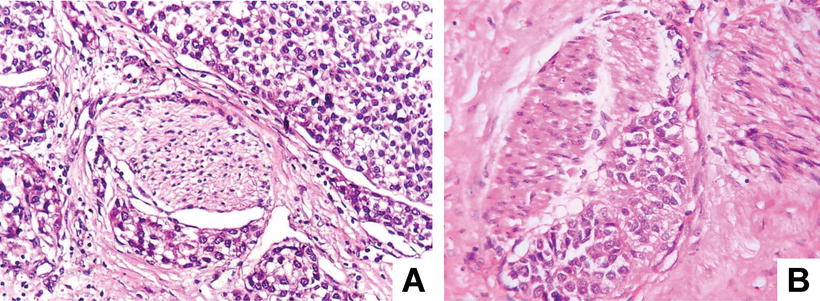
Fig. 7.90
HCC, perineural invasion of HCC cells
- 8.
Invasion of hepatic sinusoids: It is caused by the direct invasion of the carcinoma cells into the adjacent hepatic sinusoids to form map-like irregular boundaries due to the absence of capsule around the tumor (Fig. 7.91).
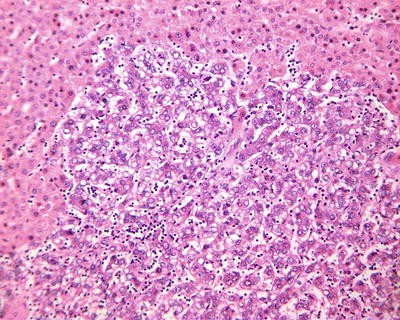
Fig. 7.91
HCC, infiltration of carcinoma cells into the hepatic sinusoids
- (5)
Inclusion body
HCC cells may contain a variety of types of inclusion bodies, which facilitate the diagnosis, and the common types of inclusion bodies mainly have the following two types.
- 1.
Pale bodies: They are oval, pale-stained, eosinophilic bodies in the cytoplasm, and the nuclei were frequently displaced to the periphery by the inclusions (Figs. 7.92 and 7.93) and negative for periodic acid-Schiff (PAS) staining and Masson’s trichrome staining. Immunohistochemistry shows they are positive for fibrinogen and electron microscope demonstrated pale bodies containing electron density particles wrapped by a membrane closely related to the dilated rough endoplasmic reticulum, suggesting that pale bodies may be caused by deficient transportation of fibrinogen resulting in its accumulation in cystic endoplasmic reticulum. The occurrence of pale bodies in the HCC cells is of diagnostic significance to some extent.
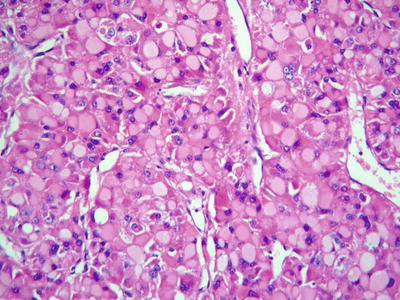
Fig. 7.92
HCC, pale bodies, tumor cells contained intracytoplasmic ground-glass-like pale bodies
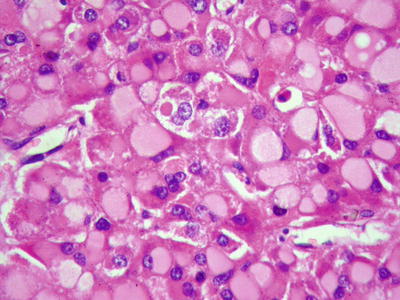
Fig. 7.93
HCC, pale bodies, hepatocytes displayed pale or eosinophilic staining of the cytoplasm with nuclei displacement to the periphery
- 2.
Eosinophilic bodies: They are spherical or rod-shaped, homogeneous red-dyed hyaline bodies in the cytoplasm, 2–20 μm in diameter. Small ones are only half the volume of a nucleus, while large ones are 2–3 times larger than a nucleus. The cytoplasm is filled with eosinophilic bodies which are surrounded by halos (Figs. 7.94 and 7.95). They are negative for α1-antitrypsin, CK, and PAS staining, containing components of p62 protein which can enhance the transcriptional activity of liver cells. The major difference between them and Mallory bodies is that the former almost does not contain ubiquitin [21].
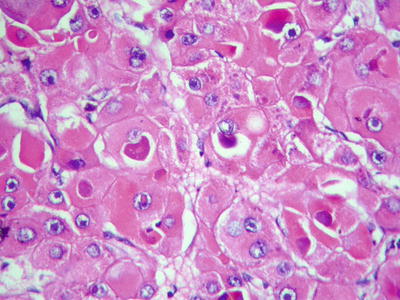
Fig. 7.94
HCC, eosinophilic bodies, intracellular hyaline bodies with surrounding halo
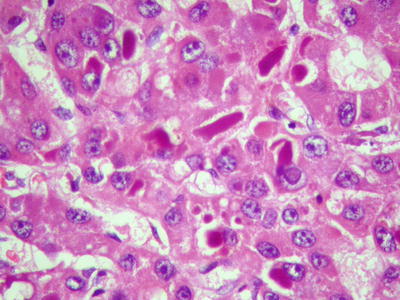
Fig. 7.95
HCC, eosinophilic bodies, intracytoplasmic oval homogeneous eosinophilic hyaline bodies
We observed that eosinophilic bodies reacted on HBsAg staining (Figs. 7.96 and 7.97), indicating the existence of associated protein components. Under electron microscope, eosinophilic bodies consist of homogeneous granules or reticular electronic density matrix, wrapped in the dilated rough endoplasmic reticulum or the remnants of the endoplasmic reticulum. It has been speculated that eosinophilic bodies are formed by intracellular accumulation of proteins due to abnormal protein secretion or transportation, which is indicative of injured tumor cells [22].
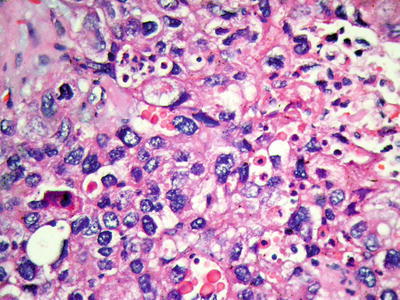
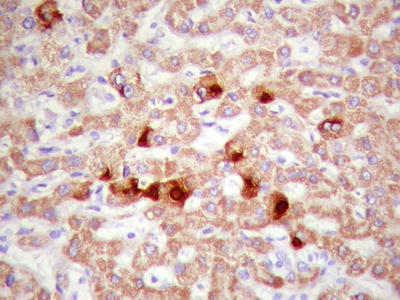

Fig. 7.96
HCC, eosinophilic bodies, intracytoplasmic small round eosinophilic hyaline bodies

Fig. 7.97
HCC, eosinophilic bodies are positive for HBsAg immunohistochemical staining
7.1.1.7 Immunohistochemistry
Commonly used markers for liver cells are Hep Par-1, CD10 (Fig. 7.98), pCEA (Fig. 7.99), GPC-3, CD34 (Figs. 7.21 and 7.24), AFP, etc. The former three markers cannot be used to distinguish the nature of liver cells, while the latter three markers are characteristically expressed in HCC tissues. And in differentiation from non-hepatocellular tumors, a combination of liver cell markers is very effective, and positive HBsAg staining is of certain reference value in the diagnosis of HCC (Fig. 7.100). The American Association for the Study of Liver Diseases (AASLD), the European Association of Liver Diseases, and the Panel of International Consensus Group all make the recommendation of diagnostic marker combination for HCC, “GPC-3+HSP70+GS,” which has a sensitivity and a specificity of 72% and 100%, respectively [23]. In addition, Yong et al. (2013) recently found high expression of the carcinoembryonic gene SALL4 in 55.6% of HCC tissues, but no expression was detected in pericarcinoma liver tissues and was associated with a poor prognosis. It can be used as a potential diagnostic marker in immunohistochemistry for HCC [24], and the evaluation of the background (inflammation, fibrosis) in the pericarcinoma liver tissue can rely on Masson staining.
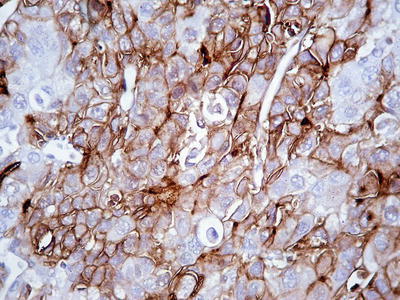
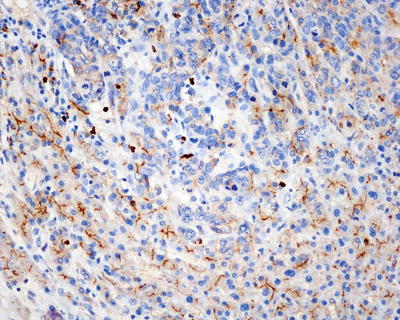
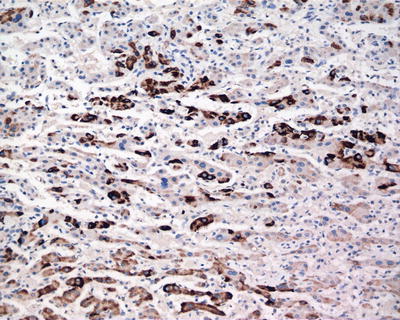

Fig. 7.98
HCC, immunohistochemical staining, a bile canalicular pattern of staining with pCEA

Fig. 7.99
HCC, immunohistochemical staining, a bile canalicular pattern of staining with CD10

Fig. 7.100
HCC, immunohistochemical staining, showing adjacent non-tumor HBsAg-positive tissue
7.1.1.8 Differential Diagnosis
The acinar (pseudoglandular)-type HCC should be differentiated from intrahepatic cholangiocarcinoma (ICC ) and hepatic metastatic adenocarcinoma (HMA), and the main differential points of the three are shown in Table 7.1. The immunohistochemical features of HCC are shown in Chap. 6 Immunohistochemistry and Special Staining for Liver Tissue.
Table 7.1
Differential diagnosisof acinar HCC, ICC , and HMA
Features | Acinar HCC | ICC | HMA |
|---|---|---|---|
Clinic | |||
Elevated serum AFP | Most cases | Some cases | Normal |
Elevated serum CA19-9 | Normal | Most cases | Normal |
Hepatitis history | Most cases | A few cases | Rare |
Histology | |||
Fibrous stroma | No/rare | Rich | Little |
Adenoid structure | Cuboidal cells | Cuboidal cells | Cuboidal/columnar cells |
Bile secretion | Yes | None | None |
Mucus secretion | None | Yes | Yes |
Immunohistochemistry | |||
Hep Par-1 positive | 83–93% | Occasionally | Occasionally |
GPC-3 positive | 50–90% | 0 | 0 |
CK19 positive | 10–20% | 85–95% | 0–40% |
MUC-1 positive | 0 | 65.8–73.8% | 50–80% |
pCEA staining | Canalicular pattern | Cytoplasmic pattern | Cytoplasmic pattern |
CD34 staining | Rich microvessels | Sparse microvessels | Sparse microvessels |
7.1.1.9 Assessment of Prognosis and Staging
Many factors have an impact on the prognosis of HCC. And to accurately evaluate the risk of postsurgical recurrence, survival, and prognosis in HCC patients, approximately 20 clinical pathological staging schemes for HCC have been proposed in the current literature, including the following HCC staging systems with great influence, such as the seventh edition of the American Joint Committee on Cancer (AJCC)/The Union for International Cancer Control (UICC) tumor-node-metastasis (TNM) staging system (Table 7.2), Okuda staging, the Cancer of the Liver Italian Program (CLIP) index, the Barcelona Clinic Liver Cancer (BCLC) score, the French staging, the Chinese University Prognostic Index (CUPI), Japan Integrated Staging (JIS), the Tokyo score, etc. The index system is usually composed of serological indexes and pathological parameters (such as tumor size, tumor number, MVI , satellite nodules, differentiation, grading, etc.), while the currently available staging systems in the literature have not received unanimous verdict in the assessment of HCC biological characteristics and prognosis of patients with HCC, each of which has advantages and disadvantages. Kee et al. (2013) identified that the seventh edition of the TNM staging was of better predictive value for the prognosis of HCC than its sixth edition [25]. In view of the high heterogeneity and malignancy of HCC, it is not sufficient to predict its complex biological behaviors based on the limited clinical and pathological indexes. Therefore, several indexes have been included into the staging of HCC, such as serum albumin, bilirubin, vascular endothelial growth (VEGF), and insulin-like growth factor-1 (IGF-1) [26]. It can be expected that, with the development of understanding on the biological characteristics of HCC, there will be consistent improvement and perfection in the prognostic evaluation system of HCC, including the international TNM staging.
Table 7.2
TNM staging of HCC by AJCC (seventh edition)
TNM staging | Features |
|---|---|
T1 | Solitary tumor without vascular invasion |
T2 | Single tumor with vascular invasion or multiple tumors, none more than 5 cm |
T3a | Multiple tumors, more than 5 cm |
T3b | Single tumor or multiple tumors of any size involving a major branch of the portal vein of hepatic vein |
T4 | Tumor with direct invasion of adjacent organs other than the gallbladder or with perforation of the visceral peritoneum |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
M0 | No distant metastasis |
M1 | Distant metastasis |
Staging | |
Stage I | T1 N0 M0 |
Stage II | T2 N0 M0 |
Stage IIIA | T3a N0 M0 |
Stage IIIB | T3b N0 M0 |
Stage IIIC | T4 N0 M0 |
Stage IVA | Any T N1 M0 |
Stage IVB | Any T Any N M1 |
In addition, Shirabe et al. (2014) proposed that in cases of HCC with the tumor diameter of 3.6 cm, the maximum standard uptake value (SUVmax) of 18F- fluorodeoxyglucose (FDG) in positron emission tomography (PET) was 4.2, and the abnormal prothrombin in serum reached 10 mAU/ml, both of which have the highest sensitivity and specificity in prediction of MVI [27]. Tsujita et al. (2012) proposed the indexes affecting the prognosis of patients with recurrent HCC: ①indocyanine green retention rate at 15 minutes, ②the disease-free interval, ③tumor size, ④portal vein invasion at resection of the primary HCC, ⑤ gender, and ⑥blood loss [28]. Kadalayil et al. (2013) proposed the prognosis indexes for TACE in HCC, including albumin <36 g/dl, bilirubin >17 μmol/L, and AFP >400 ng/ml or the diameter of the primary tumor >7 cm, each of which was recorded as 1 point. The median survival time for patients scoring 0, 1, 2, and >2 points was 27.6 months, 18.5 months, 9 months, and 3.6 months [29], respectively. Furthermore, the molecular typing of HCC will be an important trend for HCC research in the future.
7.1.1.10 Pathological Diagnosis Report
As stated, factors related to the therapeutic effect and survival of patients with HCC include growth pattern, proliferation activity, invasion and metastasis potential, postoperative recurrence risk, and variation of genes and proteins associated with biological characteristics, the main carrier for the above important information is the tumor tissue specimens. Obviously, the traditional pathology focuses on the diagnosis model mainly based on the morphological features of HCC tissues and cells; however, it does not fit the concern about the pathobiological features of HCC in modern hepatic surgery, and new adjustments and supplements in the diagnostic concept, modes, and contents should be made to establish a comprehensive mode of pathological diagnosis based on both pathology and biology, further providing practical pathological information for the improvement of clinical curative effects. This is an important trend for future pathology of hepatic tumors.
Therefore, pathological diagnosis report for HCC should meet the clinical demands and concerns for the assessment of tumor growth pattern, prediction of metastasis and recurrence risk, formulation of individualized treatment strategy and evaluation of prognosis. No uniform standards on the content or format of the pathological diagnosis report for HCC have been determined, and it mainly includes the following aspects depending on the specific circumstances:
- ①
Morphological features related to prognosis, including macroscopic (tumor number, tumor size, gross type, etc.) and microscopic features (histological types, differentiation degree, MVI , satellite nodules, growth pattern, etc.) with attached typical photographs.
- ②
Auxiliary diagnostic criteria, e.g., diagnostic and differential diagnostic evidences based on immunohistochemistry, including dual-phenotype HCC (DPHCC).
- ③
Characteristics of biological behaviors, e.g., detection results of molecular markers associated with the risk of invasion, recurrence, and prognosis of HCC.
- ④
Diagnosis of specific lesions, e.g., analysis of the clonal origin of recurrent and multinodular HCC and analysis of genomic instability in precancerous lesions , including high-grade dysplastic nodules , hepatocellular adenoma , etc.
- ⑤
Analysis on therapeutic sensitivity: Despite several molecular target drugs for HCC at present, screening of sensitive populations and specific molecular targets need further explorations. Furthermore, patients with low expression of miR-26a in HCC tissues treated by interferon were found to have an improved 5-year survival rate from 30 to 65% in the study by the Liver Cancer Institute of Fudan University, suggesting it was a potential pathological marker for evaluating interferon therapy in the future, and more attention should be paid to their further results from multicenter validation study.
- ⑥
Remarks: Specifications on important morphological and biological indicators, such as tumor invasion, metastasis and prognosis, and differential diagnosis, should be illustrated or supplemented in the remarks to facilitate clinicians’ concern and understanding.
7.1.2 Small Hepatocellular Carcinoma
- (1)
Basic concept of small hepatocellular carcinoma (SHCC )
Early discovery, early treatment, and early cure and the smaller the tumor, the better the therapeutic efficacy have always been the basic principles and the ideal goals of modern surgical oncology. Many studies have suggested that the size of solid tumors can be used as an important reference index to assess the clinical prognosis of the patients. Despite many clinical and pathological factors that affect the malignancy and prognosis of the tumor, tumor size remains the most intuitive and simple index for presurgical assessment on tumorous progression stages. A typical example is the concepts of early gastric carcinoma, small gastric carcinoma, and micro-gastric carcinoma which were proposed 30 years ago and has greatly improved the level of clinical diagnosis and treatment of gastric cancer. In the late 1970s, Chinese scholars represented by academician Prof. Zhao-You Tang and Prof. Meng-Chao Wu first put forward the important concept of SHCC , being the milestone in development of clinical research of HCC into a new era of SHCC . In those days, the understanding of SHCC features in the field of hepatic surgery mainly include the following: 70% of the HCC patients with no obvious clinical symptoms had tumors ≤5 cm in diameter, and more than 70% of the HCC patients with obvious clinical symptoms had tumors >5 cm in diameter. Therefore, the tumor volume of ≤5 cm in diameter is widely accepted as the diagnostic standard for SHCC both at home and abroad. Under the condition of limited diagnostic and treatment techniques of HCC in that time, low rates of diagnosis and surgical resection of SHCC ≤5 cm were observed, and there were even reports in which surgically excised HCC <4.5 cm was called microcarcinoma [30]. And a diameter of 5 cm was still adopted as the pathological staging criteria in the seventh edition of TNM staging of HCC issued by AJCC and UICC in 2009.
On the basis of the study of 500 autopsy cases of HCC pathological specimens, the pathological classification of the “Five Major Types and Six Sub-Types” of HCC was put forward by the Chinese Cooperative Pathology Group of Liver Cancer in 1982, and three nodules ≤3 cm in diameter were called SHCC , which was especially proposed for the first time and was one of the most prominent contributions in the field of pathology of HCC in China. Since then, we have conducted systematic studies on the relationship between HCC size and its pathological biological behaviors based on the rat carcinogenesis model and surgically excised human HCC of different tumor sizes, demonstrating that SHCC ≤3 cm is often DNA diploid dominant, with relatively benign biological behaviors of HCC in the early stage, good prognosis, and low recurrence rate, while LHCC >3 cm is often DNA ploidy dominant, with obvious malignant biological behaviors, high postoperative recurrence rate, and low long-term survival rate. Thus, it has been proposed that HCC which is approximate 3 cm in diameter is in a key period in which its biological characteristics transform from relatively benign nature to marked malignancy, and the early diagnosis and treatment of SHCC can lead to relatively good therapeutic effects based on its pathobiological feature of radical resectability. Right now, BCLC staging system-defined HCC in the early stage may include patients with single or up to three tumor nodules, each ≤3 cm. However, based on the clonal origin theory of multinodular HCC, we think there is a great possibility of intrahepatic metastasis for three tumor nodules ≤3 cm. Therefore, in the Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update issued by the Chinese Pathological Group of Hepatobiliary Tumor and Liver Transplantation, a single tumor with a diameter from >1 cm to ≤3 cm is defined as SHCC .
Based on the statistics of data from the Department of Pathology in our hospital, the proportion of SHCC ≤3 cm treated by surgical resection has continuously increased (Fig. 7.101), and the postoperative survival rate at 5 years was 67.8%. Interestingly, Moribe et al. (2009) detected the methylation of 12 genes in 25 cases of HCC using quantitative methylation-specific PCR (MSP), and the results showed that all the SHCC ≤3 cm contained three gene methylation of RASSF1A, SPINT2, and CCND2, and its specificity, sensitivity, and accuracy were 100%, ≥75%, and 95%, respectively [31]. Llovet et al. (2006) found a successively increasing expression of three-gene spectrum consisting of GPC-3, survivin, and LYVE1 spectrum in dysplastic nodules , SHCC ( diameter (2 ± 0.6) cm, ranged 0.9–3 cm) and LHCC, and the diagnostic accuracy of the gene spectrum in the three kinds of lesions reached 94% [32]. These two studies further suggest that there are corresponding molecular variations in the transformation from SHCC (≤3 cm) to LHCC (>3 cm) which is worth further study.
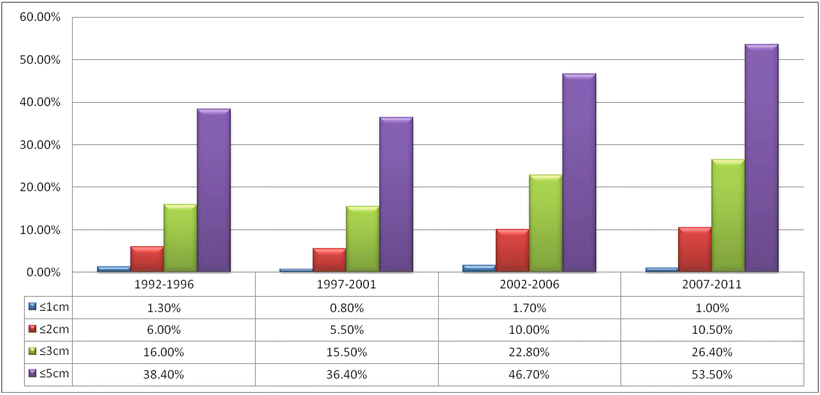

Fig. 7.101
The proportion of SHCC resection in EHBH during the period of 20 years
However, no uniform criterion for the tumor volume of SHCC has been accepted at home and abroad, and 5 cm of SHCC in diameter is still in use by many scholars, which is not correspondent to the biological characteristics of early HCC or current clinical techniques in early detection and early diagnosis of HCC. The present international staging systems, such as BCLC and AASLD, defined the early HCC as tumors ≤3 cm and very early HCC as tumors ≤2 cm. Nevertheless, up to now, most studies on SHCC ≤2 cm are based on multicenter joint studies with long-term data collection, and among the cases of surgically resected HCC in centers for hepatic diseases all around the world, reports of SHCC ≤2 cm are still very rare with only a few studies on their biological characteristics (Table 7.3). For instance, Minagawa et al. (2007) collected 2767 cases of ≤2 cm SHCC from 829 units in Japan during a 30-year period. This is a summary of a huge sample, but the actual number of cases in each year for each unit is minimal. Farinati et al. (2009) analyzed the data of 1834 HCC cases (partly confirmed by pathology) during 10 years accumulated by the Cancer of the Liver Italian Program (ITA.LI.CA) and found that cases of ≤2 cm SHCC accounted for only 3%, which could not be analyzed for internal validation due to the insufficient number of cases. Thus, the authors suggested that the tumor volume of ≤2 cm as a criterion for the diagnosis of SHCC is of less staging significance in clinical practice because these cases are few in number.
Table 7.3
Researches on SHCC ≤2 cm in the literature
Authors | Year | Number of cases | Collection period | Number of hospitals |
|---|---|---|---|---|
Kondo F et al. | 1987 | 15 | 10 years | 2 |
Nagao T et al. | 1992 | 23 | 9 years | 1 |
Nakashima O et al. | 1995 | 27 | 8 years | 1 |
Takayama T et al. | 1998 | 80 | 9 years | 2 |
Arii S et al. | 2000 | 1318a | 8 years | ≈800(LCSGJ) |
Vauthey JN et al. | 2002 | 57 | 18 years | 4个 |
Inoue K et al. | 2004 | 70 | 9 years | 2 |
Ikai I et al. | 2004 | 2320 | 10 years | ≈800(LCSGJ) |
Wu FS et al. | 2005 | 45 | 17 years | 1 |
Ando et al. | 2006 | 91a | 6 years | 1 |
Minagawa M et al. | 2007 | 2767 | 6 years | 829(LCSGJ) |
Forner A et al. | 2008 | 60a | >3 years | 4 |
Livraghi T et al. | 2008 | 218 (RFA) | ≈11 years | 5 |
Farinati F et al. | 2009 | 65a | 18 years | 10(ITA.LI.CA) |
ICGHN | 2009 | 23 (2002)b | ? | 3 |
22 (2004)b | ? | ? | ||
Takayama T et al. | 2010 | 1235 | 3 years | ≈800(LCSGJ) |
Di Tommaso L et al. | 2011 | 47(liver biopsy) | 4 years | 2 |
Yamashita Y et al. | 2012 | 149 | 16 years | 2 |
- (2)
Biological characteristics of SHCC
Many studies have been reported at home and abroad; the long-term survival rate of patients with SHCC is significantly higher than that of LHCC patients, and postoperative recurrence rate was significantly lower than that of LHCC patients. We (2011) conducted a control study of 618 cases of HCC patients, who were divided into five groups according to the diameter of the tumors, ≤1 cm, 1.1–2 cm, 2.1–3 cm, 3.1–5 cm, and >5 cm, and the results showed that no significant difference in pathologic parameters and operative prognosis is found between 1–3 cm and >5 cm groups except the group of patients with tumors ≤1 cm in diameter (Fig. 7.102). However, if the patients were divided into two groups with the tumor diameter of 3 cm as a threshold, there were significant difference in pathologic parameters and operative prognosis between the two groups (Fig. 7.103) [33].

Fig. 7.102
Comparison of the postoperative prognoses among five groups of patients with HCC. (a). Overall survival; (b). Recurrence-free survival

Fig. 7.103
Comparison of the postoperative prognoses between SHCC group and LHCC group. (a). Overall survival; (b). Recurrence-free survival
Based on our observations, the main biological characteristics of SHCC include DNA diploid dominant, relatively slow growth with single and localized nodule, high degrees of differentiation, thin-trabecular type in histology, with or without a fibrous capsule, clear boundaries, rare MVI or satellite focus which are usually found in liver tissues 0.5–1 cm from the surgical margin, high postsurgical survival rate, and low recurrence rate. Tumors larger than 3 cm in diameter have pathobiological indexes which indicate transformation into malignant LHCC in progression, such as significantly increased rates of capsular and microvascular invasion , occurrence of satellite focus, and postsurgical recurrence. Combining our researching results and understanding, the growth and progression of HCC can be generally divided into four stages correspondent to four patterns of tumor growth, including microcarcinoma (≤1 cm, non-capsule stage) with transition between the tumor margin and pericarcinoma liver tissues → SHCC (1–3 cm, capsule formation stage) with dominantly solitary nodular growth, a clear border and an intact capsule in most of the cases, or with capsular invasion in a few cases → medium-sized HCC (3–5 cm, accelerated growth stage) with more extracapsular sub-carcinoma foci and increased microvascular invasion → large HCC (>5 cm, malignant advanced stage) with multifocal infiltration in most cases, capsular invasion, MVI , and commonly seen satellite nodules (Fig. 7.104) [33, 34].
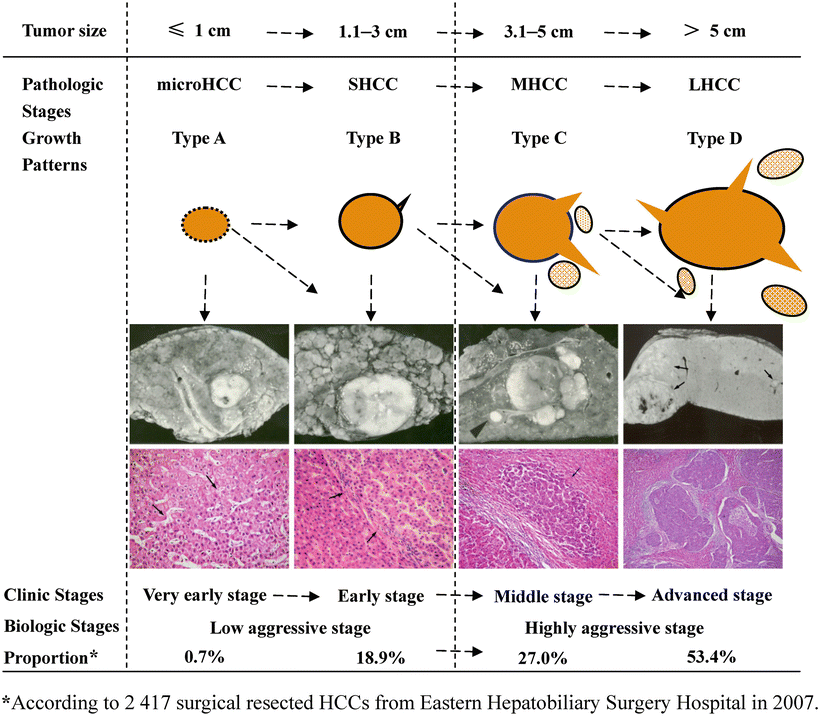

Fig. 7.104
EHBH pathological staging of HCC
- (3)
Concepts of SHCC and early HCC
Early HCC (EHCC) should be referred to a small and well-differentiated HCC in the early stage of development, with relatively benign biological behaviors, no vascular invasion, and intrahepatic metastasis. However, the current conception and criterion of EHCC and SHCC is not consistent in the world. In BCLC staging classification, three nodules ≤3 cm in diameter were included in the early stage (stage A). However, from the point of view of pathology, three HCC nodules have a great possibility of intrahepatic metastasis, suggesting not in early stage in a biological sense. Nathan et al. (2013) defined EHCC as HCC ≤5 cm in diameter, without metastatic foci, lymph node metastasis, extrahepatic expansion, or invasion of major blood vessels. Sakamoto et al. (1998) defined EHCC as well-differentiated (Edmondson pathological grade I or grade I with grade II in a few region) HCC with negative tumor staining in angiography regardless of the size of the tumor. EHCC was also adopted in the WHO’s Pathological Classification of Hepatobiliary Tumors (2010), which was defined as well-differentiated SHCC with a diameter of <2 cm. In general, SHCC refers to a HCC in small size, which is a morphological concept, while EHCC refers to HCC in early stages of tumorigenesis and progression with relatively benign biological behaviors, which is a biological concept. Therefore, it is inappropriate to simply equate SHCC and EHCC, since a few cases of SHCC have malignant biological behaviors including DNA ploidy, capsular invasion, and MVI formation in earlier stages. Table 7.4 shows the differences in criteria of staging systems on SHCC and EHCC in the literature, each of which has a certain influence.
Table 7.4
Criteria on SHCC and EHCC in the literature
Staging system by | Year | Staging | Tumor diameter |
|---|---|---|---|
CSLC | 2001 | Ia | ≤3 cm |
Ib | ≤5 cm | ||
IIa | ≤10 cm | ||
IIb | >10 cm | ||
BCLC | 2003 | Very early HCC | <2 cm |
Early HCC | 1or 3 nodules, ≤3 cm | ||
IHPBA | 2003 | T1 | ≤2 cm |
Llovet et al. | 2006 | Very early HCC | ≤2 cm, MVI (−) |
Early HCC | ≤2 cm, MVI (+),or | ||
2–5 cm, or | |||
2–3nodules, ≤ 3 cm | |||
LCSGJ | 2007 | T1 | ≤2 cm, MVI (−) |
AJCCTNM staging (seventh edition) | 2009 | T1 | Any size, MVI (−) |
T2 | Any size, MVI (+) | ||
2009 | Early HCC | <5 cm | |
JSH | 2010 | Early HCC (SHCC ) | ≤3 cm, well-differentiated |
- (4)
Clinicopathological significance of SHCC
When evaluating the biological characteristics according to the tumor size of HCC, most SHCC ≤3 cm presents relatively benign performances of EHCC in early stage of tumorigenesis and development, with long-term survival rate and low recurrence rate after radical resection, indicating SHCC ≤3 cm has the pathological basis of radical resectability. However, we have found that about 26% of the SHCC contain DNA ploidy, presenting significantly malignant biological behaviors, such as poor differentiation, capsular invasion, and formation of microvascular thrombus, with postsurgical early recurrence; even a microcarcinoma of 0.6 cm in diameter may contain MVI , suggesting that these SHCC have an increased growth activity, and has an accelerated progress of malignancy, which does not belong to the biological category of EHCC. It is certain that part of the LHCC may have the morphological representation of SHCC and the biological characteristics of EHCC, with long-term survival rate and low recurrence rate after radical resection. Thus, SHCC is not completely equal to EHCC in the strict sense. In clinical surgery, the individualized therapeutic strategy for SHCC should be formulated according to the pathological type and growth pattern with a certain extent of surgical resection. Generally, for SHCC ≤1–3 cm in diameter, a retaining resection extension of 0.5–1 cm should be considered, and for HCC larger than 3 cm, it should be >1–2 cm to completely remove possible residual satellite foci or MVI , which also fits in radiofrequency ablation in order to improve the long-term treatment efficacy.
- (5)
Pathological characteristics of SHCC
7.1.2.1 Gross Features
SHCC is often a solitary nodule in expansive growth. The section of SHCC shows homogeneous and dense tissue, with visible slender radial fibers, similar to the hepatocellular tumor-like nodules, and a clear boundary separating it from the surrounding liver tissue (Fig. 7.105). It can also be in multinodular fusion growth (Fig. 7.106), and approximately 67% of SHCC cases have a complete fibrous capsule. Some SHCC or microcarcinomas have no obvious capsule or continuous capsule, with expansive extrusion boundary, suggesting the capsule is a local defensive reaction during the growth and expansion of the tumor. However, satellite foci and tumor thrombus can be found on the cutting margin of the tumor (Figs. 7.107 and 7.108), and these invasive behaviors become more significant as the tumor increases in size in most cases, especially in LHCC with a diameter>3 cm, in which the occurrence rate of invasion is higher.
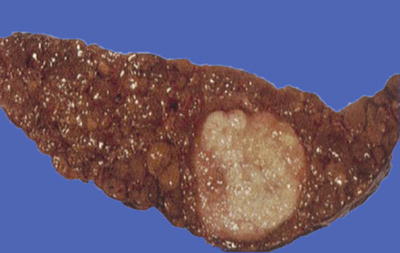
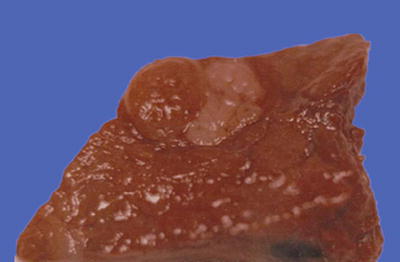
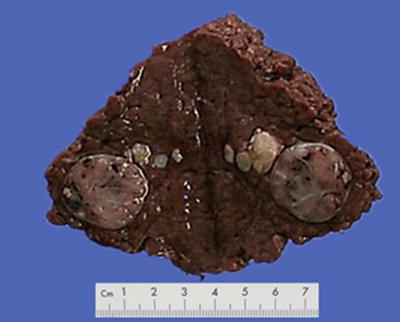
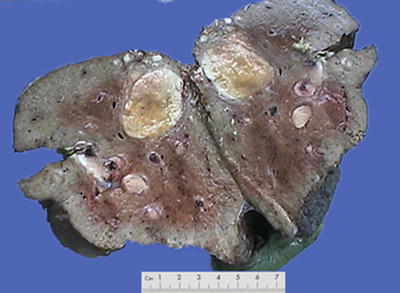

Fig. 7.105
SHCC , a round tumor nodule with clear boundary

Fig. 7.106
SHCC , two nodules become confluent with clear boundary

Fig. 7.107
SHCC , several satellite foci in the surrounding cancer tissue

Fig. 7.108
SHCC , multiple satellite foci in the surrounding cancer tissue
7.1.2.2 Microscopic Features
Approximately 60% of SHCC are well differentiated in grade I to grade II (Figs. 7.1, 7.2, 7.3, 7.4, 7.5, 7.6, 7.7, 7.8, 7.9, 7.10, 7.11, 7.12, 7.13, 7.14, 7.15, 7.16, 7.17, 7.18, 7.19, and 7.20), and the cancer cells are consistent in size, with slight nuclear atypia, increased cell density, eosinophilic or basophilic cytoplasm, darker-stained nucleus, slightly increased nuclear to cytoplasmic ratio, and mitotic figures in a few cases. The tumor cells are often arranged in thin trabecules, and the hepatic sinusoid gap increases, with or without pseudoglandular structures. Infiltration of a large number of lymphocytes in the tumor boundary or tumorous stroma (Figs. 7.109 and 7.110) suggests a strong focal immune response in the patient. Portal tracts can also be found in some microcarcinoma, the number of which decreases with the increase of HCC volume, while the number of arterioles increases with the increase of volume of HCC, reflecting the dynamic changes of blood supply pattern during the growth of HCC, and abnormal blood supply in this process may be an important factor leading to high differentiated SHCC , especially microcarcinoma with frequent fatty changes (Fig. 7.111). It should be noted that SHCC with capsular invasion or even invasion into the surrounding liver tissue is not a rare phenomenon (Fig. 7.112) and should be paid careful attention in observation.
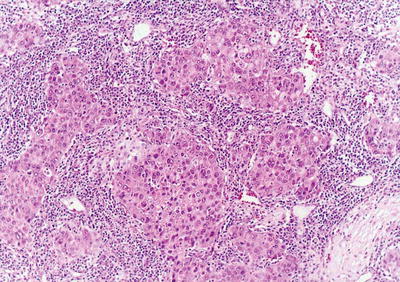
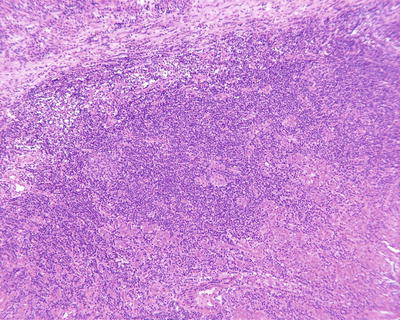
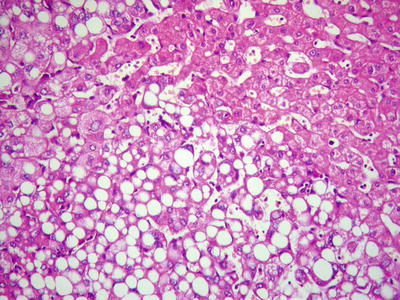
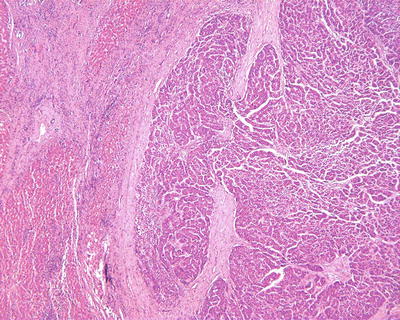

Fig. 7.109
SHCC , large amounts of lymphocytes infiltrated in the tumorous stroma

Fig. 7.110
SHCC , a large number of lymphocytes infiltrated at the peripheral region

Fig. 7.111
SHCC , steatosis of liver tumor cells

Fig. 7.112
SHCC , capsular invasion and surrounded by fibrous membrane
7.1.2.3 Differential Diagnosis
Due to the complete envelope in most cases of well-differentiated SHCC or transition between the tumor and the liver tissue without obvious invasion of adjacent liver tissue, it should be well differentiated from hepatocellular nodular lesions, such as hepatocellular adenoma (HCA), high-grade dysplastic nodule (HGDN) , hepatic focal nodular hyperplasia (FNH), etc. Cases with obvious fatty degeneration should also be distinguished from hepatic epithelioid angiomyolipoma . Much difficulty exists in the differential diagnosis of small liver biopsy tissues, and examinations such as immunohistochemistry or gene marker detection are beneficial. It is worth noting that well-differentiated SHCC can exhibit untypical “HCC-like microvascular density” in CD34 staining (Fig. 7.113) which may interfere the judgment, and reticular fiber staining is helpful to distinguish normal liver cells and tumor cell nests interspersed in the interstitial fibrous trabecules. The absence of local bile duct reaction in CK7 staining in the surrounding fibrous tissue around the liver cell mass may suggest the stromal invasion of a true tumor. The expression intensity in Ki-67 or PCNA staining (Fig. 7.114) contributes to the assessment of the nature of the tumor. Tremosini et al. (2012) conducted an ultrasound-guided fine needle aspiration biopsy in 60 cases of hepatic nodular lesions with diameter of 5–20 mm, and the tissues are examined using a combined immunohistochemical staining panel of “GPC-3+HSP70+GS”; the result of which with positive staining of two markers facilitates the diagnosis of HCC with sensitivity and specificity of 62% and 100% [35], respectively. However, according to our experience, similar to reports of some other scholars, the sensitivity of GPC-3 for HCC is relatively low (<50%), especially in cases with negative GPC-3 staining, and should be carefully diagnosed. In 2009, 34 famous pathologists from 13 countries and 2 clinicians formed the International Consensus Group for Hepatocellular Neoplasia (ICGHN), and they put forward the consensus of pathodiagnosis of early HCC after 7 years’ work, and interstitial infiltration was included as a major criterion for the diagnosis of EHCC or differential diagnosis from high-grade dysplastic nodules [23].
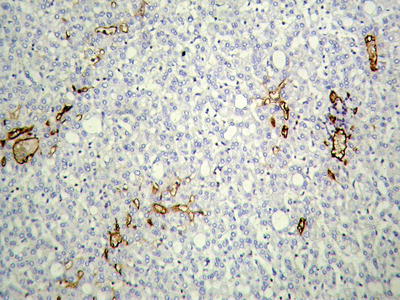
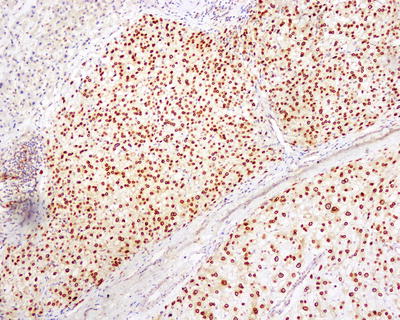

Fig. 7.113
SHCC , immunohistochemical staining of CD34 showing microvascular rarefaction

Fig. 7.114
SHCC , immunohistochemical staining of PCNA showing strong positive staining of the nuclei
7.1.3 Fibrolamellar Hepatocellular Carcinoma
7.1.3.1 Pathogenesis and Mechanism
Fibrolamellar hepatocellular carcinoma (FL-HCC) was first reported by Edmondson in 1956. Regional differences have been found in the occurrence rates of FL-HCC. It accounts for 1–5% of HCC and is rare in endemic areas of HCC with extremely rare cases found in China. The incidence rate of FL-HCC in the United States is 0.02/100,000 [36]. The expression of TGF-β in FL-HCC cells reaches as high as 82%, which is probably associated with characteristic lamellar fibers, and FL-HCC cells also express cholangiocyte markers such as EMA and CK7, as well as the stem cell markers EpCAM and CK19, suggesting that FL-HCC may derive from hepatic progenitor cell with ductular differentiation potentials. FL-HCC does not contain mutation of CTNNβ or p53 gene, and high expression of Y654-catenin is a suggestion of upregulation of the receptor tyrosine kinase, while some people consider FL-HCC as an independent disease. FL-HCC has not been found to be caused by certain factors or combined with other diseases, and little association was found between it with common factors related to HCC, such as HBV/HCV hepatitis, cirrhosis of the liver, alcohol, or metabolic diseases. Honeyman et al. (2014) conducted whole transcriptome and genome sequencing in FL-HCC and found that in all 15 cases of FL-HCC tissue detected DNAJB1-PRKACA chimeric transcripts, which may be associated with the pathogenesis of FL-HCC but not in conventional HCC and could also be a potential-specific molecular marker in support of the diagnosis of FL-HCC [37].
7.1.3.2 Clinical Features
Typical cases of FL-HCC are often found in adolescents and young adults, aged 5–35 years old (85% at age 35 years or younger) with slightly more women. The clinical symptoms are similar to those of common HCC but often with normal liver function, no history of HBV/HCV infection, and no background of liver diseases such as hepatitis and cirrhosis. The serum AFP is negative in 85–90% of the patients, but serum CEA can be increased. Elevation of serum vitamin B12 and capacity of unsaturated B12 binding, transcobalamin, and neurotensin can be discovered in most FL-HCC cases with a relative specificity. Dynamic CT scanning shows lobulated masses with heterogeneity and central scars in the normal livers, and MRI T2-weighted images show low density with calcification in most cases. In fact, FL-HCC is extremely rare in China or even in Asia. In the study of Malouf et al. (2014), the statistics of 90 cases of FL-HCC in the United States showed that Asians accounted for only 4.4% of the patients [38].
So far, only five cases of typical FL-HCC both clinically and pathologically have been diagnosed in the Department of Pathology of EHBH, Second Military Medical University, and the patients were ages 21.2 years old on average with a male to female ratio of 0.66. The patients had no history of hepatitis, and negative or weakly positive serum AFP was found in 80% of the patients with the mean tumor diameter reaching 13.2 cm (Table 7.5). And the four consultation cases of FL-HCC diagnosed in our hospital concerned patients with a male to female ratio of 1:1 and the average age of 26.8 years old.
Table 7.5
Clinical and pathological characteristics of five cases of FL-HCC
Case | Gender | Age (years) | Reasons for admission | HBV/HCV infection | Serum AFP(μg/ml) | Location | Size (cm) |
|---|---|---|---|---|---|---|---|
1. | Female | 14 | Abdominal pain | Negative | >1000 | Left lobe | 19 × 16 |
2. | Female | 26 | Health check | Negative | 4.2 | Right lobe | 7 × 5.6 |
3. | Female | 32 | Abdominal pain | Negative | 8.6 | Right lobe | 15 × 13 |
4. | Male | 20 | Health check | Negative | 1.2 | Right lobe | 11.7 × 9.3 |
5. | Male | 14 | Abdominal mass | Negative | 33.4 | Right lobe | 13.1 × 12 |
7.1.3.3 Gross Features
The lesions are located in the left hepatic lobe in more than 50% of the FL-HCC cases, which are often huge in volume with an average diameter of 13 cm ranged 3–25 cm, and great depression can be found in the center due to contraction of the fibrous scar (Case 1, Fig. 7.115). It has been reported resected FL-HCC of 40 cm in diameter and 6000 g in weight, and the patient remains survived 14 years after the surgery. The section of the tumor contained a central or eccentric radial fibrous scar, separating the FL-HCC tissue into multinodular lobulated structure (Case 3) (Case 5, Fig. 7.116), often with an intact capsule and are green in color because of the bile content. Cystic degeneration can be found in huge lesions, and the surrounding liver tissue is often without chronic hepatitis or cirrhosis.

Fig. 7.115
FL-HCC, showing central scar and central depression

Fig. 7.116
FL-HCC, showing central stellate scar on the cut surface
7.1.3.4 Microscopic Features
FL-HCC is morphologically characterized by laminated fibrous layers interspersed between the oncocytic polygonal cells. The cancer tissue was arranged in nests or trabecules and surrounded by abundant and dense lamellar fibrous tissue in an orderly form containing collagen of type I, type III, and type V. The cancer cells are often polygonal, large in volume, with obvious and hawkeye-shaped nucleolus, few mitotic figures, strongly eosinophilic granular cytoplasm, and a large number of swelling mitochondria; thus, it is also called eosinophilic HCC with lamellar fibrosis (Figs. 7.117, 7.118, 7.119, and 7.120). The cytoplasm often contains anti-amylase digestion and positive PAS-stained eosinophilic bodies (Fig. 7.121), and about 50% of the cases contain pale bodies (Fig. 7.122) which composed by α1-AT and fibrinogen and are negative for PAS staining and positive for ubiquitin antibody staining. Mucus staining shows the mucus produced by FL-HCC in some cases, which is significantly different from conventional HCC. Rhodanine staining shows that most FL-HCC has accumulation of copper-binding proteins. The tumors usually have a complete fibrous capsule on the margin of them.
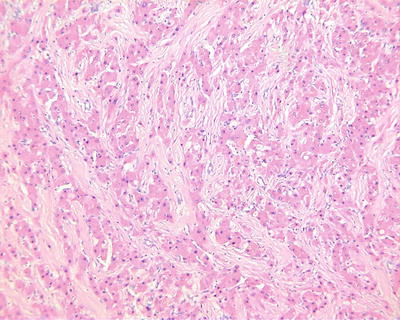
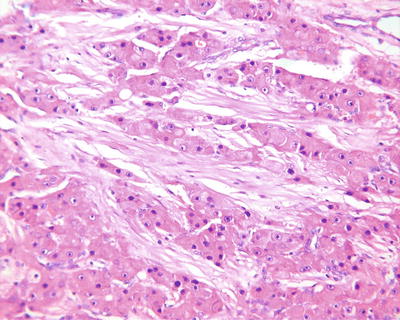
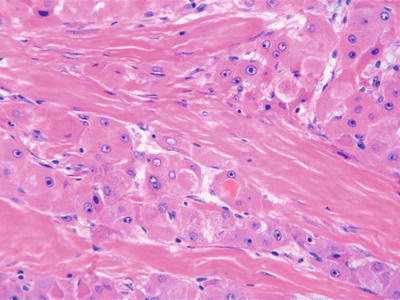
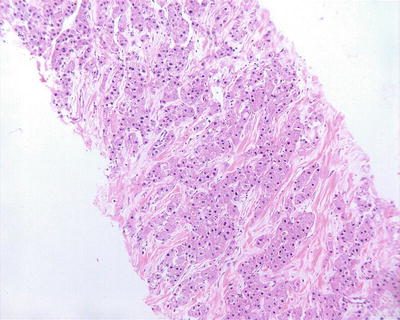
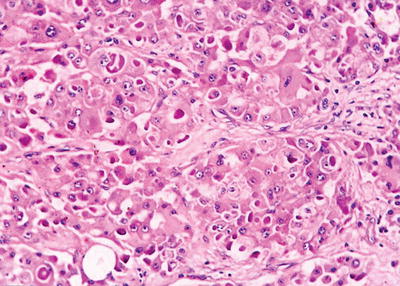
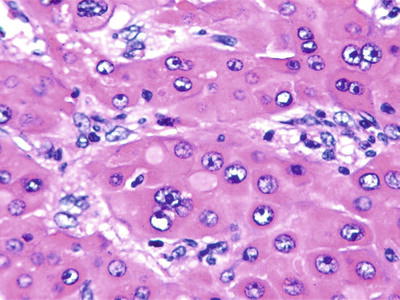

Fig. 7.117
FL-HCC, polygonal cells with granular eosinophilic cytoplasm surrounded by lamellar fibrous bands

Fig. 7.118
FL-HCC, showing thick fibrous collagen bands in a unique “lamellar” pattern

Fig. 7.119
FL-HCC, fibrous collagen bands encircling or surrounding the neoplastic hepatocytes

Fig. 7.120
FL-HCC, liver biopsy, showing abundant fibrous collagen bands

Fig. 7.121
FL-HCC, showing cytoplasmic eosinophilic bodies

Fig. 7.122
FL-HCC, showing cytoplasmic pale bodies
7.1.3.5 Immunohistochemistry
FL-HCC expresses hepatocellular markers, such as Hep Par-1, pCEA, GPC-3 (Fig. 7.123), CK8 and CK18, as well as bile duct markers CK7 and CK19 (Fig. 7.124). In addition, they are often positive for α1-AT, fibrinogen, C-reactive protein and ferritin staining, and negative for HBsAg staining. CD34 staining displays dense micro-blood vessels. Also, the FL-HCC tissues are found to express neuroendocrine markers, such as Syn, CgA, and NSE. And Malouf et al. (2014) recently found that FL-HCC specifically expressed neuroendocrine protein PCSK1 which was diffusely distributed in the cytoplasm and helpful in diagnosis.
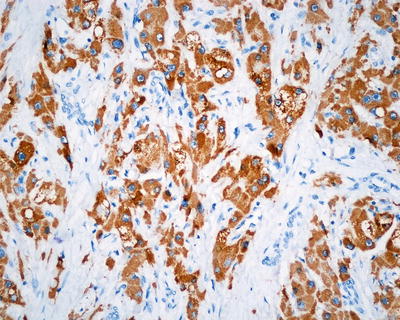
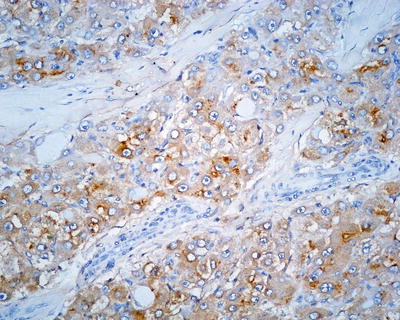

Fig. 7.123
FL-HCC, showing positive immunohistochemical staining of Hep Par-1

Fig. 7.124
FL-HCC, showing positive immunohistochemical staining of GPC-3
7.1.3.6 Electron Microscopic Observation
It is characterized by the emergence of a large number of back-to-back mitochondria, as well as intracytoplasmic globular hyaline bodies, Mallory bodies, and occasional neurosecretory granules.
7.1.3.7 Differential Diagnosis
FL-HCC should be carefully distinguished from sclerosing or scirrhous HCC. The main differential points between them have been summarized in Table 7.6. Cheuk et al. (2001) reported one case of clear cell-type FL-HCC, but it was negative for both PAS staining and post-diastase digestion PAS staining, suggesting it did not contain glycogen to exclude the diagnosis of clear cell-type HCC.
Table 7.6
Clinicopathological characteristics of FL-HCC and conventional HCC
Features | FL-HCC | HCC |
|---|---|---|
Average age (years) | 20–25 | 55–60 |
Male to female ratio | 1:1 | (4–8):1 |
Liver cirrhosis | None | Common |
Serum HBsAg | None | Common |
Serum AFP | Negative (most cases) | Positive (30–85%) |
Serum vitaminB12 | Elevated | Normal |
Serum neurotensin | Elevated | Normal |
Radial fibrous scar | Common | None |
Capsular invasion | Rare | Common |
Cholestasis | Common | Yes |
Fibrolamellar structure | Yes | None |
Eosinophilic cytoplasm | Yes | None |
Pale bodies | Common (50%) | Few (<10%) |
Tissue calcifications | Common | Few |
CK19 positive | 23% | 5–10% |
Surgical resection rate | 48–75% | 10–20% |
Five-year survival after surgery | ~75% | ~30% |
7.1.3.8 Treatment and Prognosis
Most of the researchers found that the FL-HCC has a high rate of surgical resection and a better prognosis than that of conventional-type HCC, with a 5-year survival rate reaching 30–76%. Younger age, no history of liver diseases, and early stage of tumor may be the important factors for a better prognosis. Kaseb et al. (2013) proposed combined therapy of fluorouracil and interferon was an effective treatment for FL-HCC, which could prolong the survival period of the patients [39]. However, the rate of local lymphatic metastasis in FL-HCC is as high as 40%, and these patients have a poorer prognosis. A study of European Childhood Liver Tumors Strategy Group (SIOPEL) suggested that there was no significant difference of 3-year overall survival rate between FL-HCC and conventional-type HCC (42%:33%) [40].
7.1.4 Combined Hepatocellular-Cholangiocarcinoma
7.1.4.1 Pathogenesis and Mechanism
Combined hepatocellular-cholangiocarcinoma (cHCC-CC) is defined as a single tumor composed of both unequivocally HCC and intrahepatic cholangiocarcinoma (ICC ) components, also known as hepatocholangiocarcinoma. It has been reported that regional difference exists in the incidence of cHCC-CC in the literature, which ranges from 1.4 to 6.5% accounting for 0.4–14.2% of hepatic tumors. Of all the malignant hepatobiliary tumors diagnosed in our department during the 30 years, cHCC-CC accounted for about 1.67%. Garancini et al. (2014) reported a group of American data and displayed that cHCC-CC accounted for 0.77% of HCC [41].
The histogenesis of cHCC-CC is still not very clear with current hypotheses including the following:
- 1.
Collision tumor: Two independently developed HCC and ICC nodule gradually move closer and eventually merge into one tumor in their growth process. The incidence of liver collision tumor is 0.1–1%. And it is characterized by binocular fusion tumor in morphology with a fibrous capsule separating the two kinds of tumor components, without confusion or transition. And the two components belong to two independent cell clones of different genetic phenotype. However, the so-called collision tumor of the liver is not included in the definition of cHCC-CC proposed by WHO classification.
- 2.
It is generated by differentiation of HCC or CC to another component, due to the finding of intermediate transitional zone between HCC and CC components [42]. But even the degrees of differentiation of cancer cells in the intermediate transitional zone have no significant difference from the surrounding HCC and ICC tissues. In addition, as a mature component of HCC and ICC , the actual incidence rate of cHCC-CC will be a little higher if there is a path for the tumor to transform into another type of tumor via dedifferentiation or transdifferentiation.
- 3.
Bidirectional differentiation of hepatic progenitor cells. Coulouarn et al. (2012) found that two main signaling pathways are activated in cHCC-CC tissues, namely, transforming growth factor-β (TGF-β) and Wnt/β-catenin pathway, and the genetic spectrum variation characteristics are similar to those features of HCC with characteristics of stem cells, including low differentiation degree and poor prognosis, suggesting there is a continuous development among ICC , cHCC-CC , and poorly differentiated HCC [43]. Cai et al. (2012) suggested that HBV infection-induced chronic liver inflammation could activate hepatic progenitor cells, the activity of which was related to the invasion of cHCC-CC and the proliferation of pericarcinoma small bile ducts, and the latter is a useful marker for predicting the risk of recurrence [44]. We had studied both components of the tumor in 16 cases of cHCC-CC via comparison analysis of microsatellite loss of heterozygosity (LOH) frequency in order to investigate the clonal relationship between them. The results showed that the difference rate of LOH model in all the cases of cHCC-CC was less than 30%, indicating that they were both derived from the same clone.
7.1.4.2 Clinical Features
The clinical manifestations, imaging features, and biological characteristics are between those of cHCC-CC and HCC, which are further associated with the proportion of HCC and ICC in the tumor tissues. If the proportion of HCC is larger, the patients will manifest more characteristics of HCC and vice versa. During the 30 years, 563 cases of cHCC-CC have been diagnosed in the Department of Pathology of EHBH with a male to female ratio of 4.86:1 and a median age of 50 years old, and the infection rate of HBV was 76.7%. The typical CT images of cHCC-CC show early enhancement in the peripheral region around the tumor and delayed enhancement of the central region with slight enhancement in peripheral areas. Generally speaking, cases with characteristic images of HCC with elevated serum CA19-9 and cases with characteristic images of ICC with elevated serum AFP, or with both elevation of serum AFP and CA19-9, are possibly cHCC-CC , but pathological examination is necessary to confirm the diagnosis.
7.1.4.3 Gross Features
cHCC-CC is similar to the characteristics of conventional HCC; however, tissue in different texture and color can sometimes be observed, and localized sampling can show different parts characterized by the features of HCC and ICC (Figs. 7.125 and 7.126).
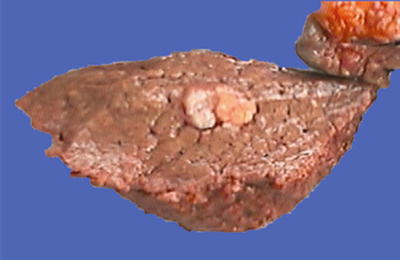
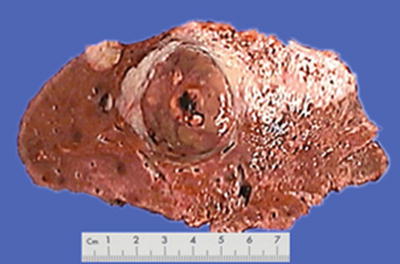

Fig. 7.125
cHCC-CC , no fibrous separation between ICC (nodule with gray-white color, left) and HCC (pale-yellow area, right)

Fig. 7.126
cHCC-CC , no fibrous separation between HCC (central brown area) and ICC (gray-white area around the tumor periphery)
7.1.4.4 Microscopic Features
- (1)
Histological classification
- 1.
Allen-Lisa (1949) classification: Type A, characterized by synchronous, separate, and autonomous epicenters of HCC and CC in one liver; type B, comprised of closely admixing distinguished foci of HCC and CC; and type C, consisting of truly combined HCC and CC components originating from the same tumor.
- 2.
Goodman (1985) classification: Type 1 (collision tumor), synchronous or metachronous occurrence of distinct epicenters of HCC and CC in the same liver, which collide each other; type II (transitional tumor), intimate intermingling of two components with actual transition of HCC elements to CC elements in the same tumor; and type III (fibrolamellar tumor), similar to a fibrolamellar HCC but with mucin-producing pseudoglands.
- 3.
WHO (2010) classification: A tumor contains two unequivocal, intimately mixed components of both HCC and CC which only consist of type C in Allen-Lisa classification and type 2 in Goodman classification. It can be divided into two types: ① classical type consists of HCC and ICC , as well as the transition zone between them, and cancer cells in the transition zone have intermediate morphology of liver cells and bile duct cells, similar to stem cells or progenitor cells and ② tumors with the characteristics of stem cells. From the perspective of the WHO criteria as well as the reports in the literature, the stem cell components in the cHCC-CC are mostly distributed in local or marginal parts of the tumors. Furthermore, the HCC and CC themselves have the ability to differentiate into neuroendocrine tissues, and there are reports of collision tumor composed by HCC and neuroendocrine tumors [45, 46]. So, the classification of cHCC-CC with stem cell features should also be consistent with the definition of cHCC-CC by WHO, and further studies are needed to clarify the classification significance of cHCC-CC with stem cell features.
It is worth noting that cholangiocellular carcinoma was once considered as a subtype of ICC ; however, WHO described and discussed it as a stem cell subtype of cHCC-CC which can often been discovered in the peripheral regions of the tumor [4]. Based on our own experience, carcinoma of small bile ducts mainly contains tumorous small bile ducts with or without few small HCC nest; thus, actually it does not meet the morphological diagnostic criteria of cHCC-CC by WHO. To understand more on the above condition, more pathological data and in-depth study on the carcinoma of small bile ducts are further needed. And carcinomas of small bile ducts are discussed specifically in the chapter of ICC in this book [47–50].
- (2)
Histological morphology
The basic feature of cHCC-CC is the defined coexistence of two tumor components of HCC and CC in a liver nodule, each of which expresses its own immunohistochemical phenotype, and both tumorous components can be separated in different regions (Figs. 7.127 and 7.128) or mingled (Figs. 7.129 and 7.130), with no real fibrous capsule to separate them. HCC components are trabecular, pseudoglandular, or dense in structure, with polygonal cancer cells, less interstitial tissue, and lining of sinusoid blood spaces between trabecules, while CC components are associated with gland-like structures, secretion of mucus, lining of cuboidal cancer cells, abundant interstitial fibrous tissue, and transition between cHCC-CC components can often be observed, though the cancer cells in the transition (intermediate type) may not have the typical morphological and histological features of HCC or CC cells.
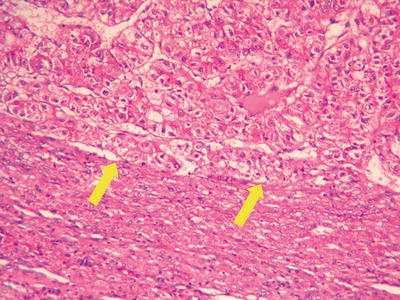
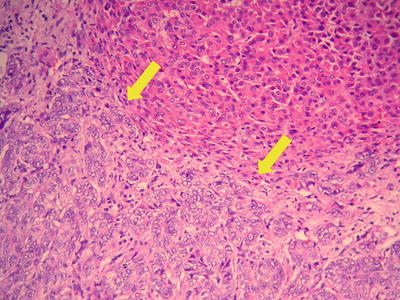
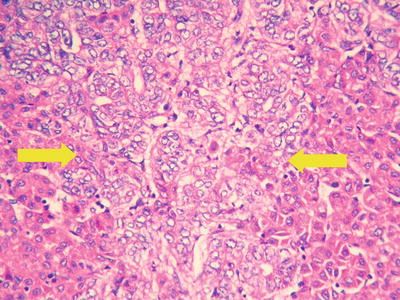
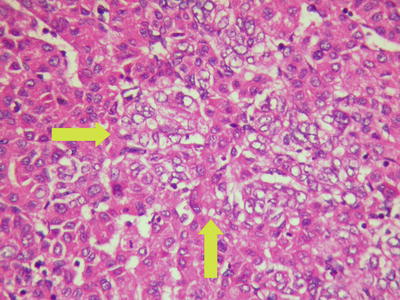

Fig. 7.127
cHCC-CC , ICC (upper) and HCC (below) closely adjacent

Fig. 7.128
cHCC-CC , HCC (upper) and ICC (below) closely adjacent

Fig. 7.129
cHCC-CC , ICC (central area) and HCC (peripheral area) closely adjacent

Fig. 7.130
cHCC-CC , ICC (arrow) intertwined with HCC components
There is no definite proportion of the two components in cHCC-CC currently. And to standardize the diagnostic criteria, according to our experience, it should be taken into account that sampling bias and lesion distribution with heterogeneity cannot be totally avoided; the proportion of one of the two tumorous components in cHCC-CC should reach 30–50% or above. For cases with a tumor component accounting for 15–30%, it is determined that this component is the dominant one in cHCC-CC tissues in pathological diagnosis, and if a tumor component is less than 15%, it should be considered to increase the sampling sites and quantity to determine whether it is a true cHCC-CC , or the dominant component should be considered as an independent lesion, and the proportions of the rest should be recorded and illustrated, because these lesions often cannot reflect the unique biological characteristics of cHCC-CC as an independent disease.
7.1.4.5 Immunohistochemistry
cHCC-CC is often characterized by biphasic phenotype, namely, regions of HCC are positive for Hep Par-1 (Fig. 7.131) and GPC-3, with or without staining of CK19 (Fig. 7.132) and CK7 (Fig. 7.133), while CC parts are positive for CK19 (Fig. 7.132), CK7 (Fig. 7.133), and MUC-1, with or without Hep Par-1 staining (Fig. 7.131). The cancer cells in the transition zone can also be examined by CD10 and polyclonal CEA staining, to determine the existence of hepatocellular or cholangiocellular differentiation in these cancer cells. From the perspective of immunopathological typing, staining of stem cell markers can also be an option, such as CD56, c-kit, CD133, or EpCAM.
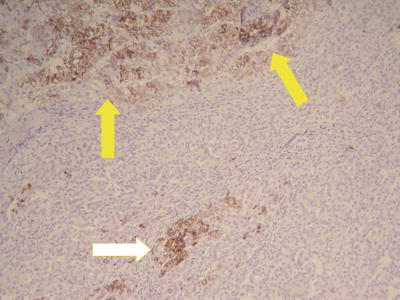
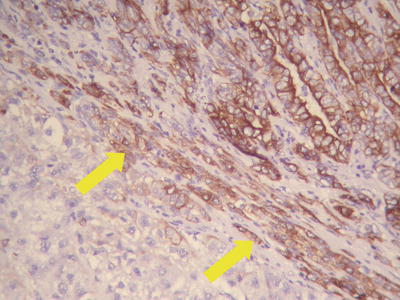
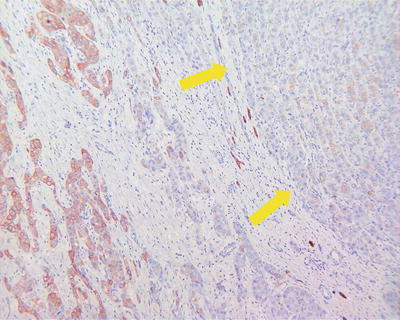

Fig. 7.131
cHCC-CC , showing positive immunohistochemical staining of Hep Par-1 in HCC area (upper yellow arrow) and ICC area (white arrow)

Fig. 7.132
cHCC-CC , showing positive immunohistochemical staining of CK19 in ICC area (upper right) and intermediate cells (arrow)

Fig. 7.133
cHCC-CC , showing positive immunohistochemical staining of CK7 in ICC (below left) and weak staining of CK7 in HCC cells (upper right)
7.1.4.6 Differential Diagnosis
The diagnosis of cHCC-CC should the based on the combination of histological and immunohistochemical features of the tumor tissues, and we should pay attention to identification of pseudoglandular tube structures in the HCC tissues from true glandular tubes of CC, as well as collision tumor produced by fusion growth of HCC and CC (Figs. 7.134 and 7.135).
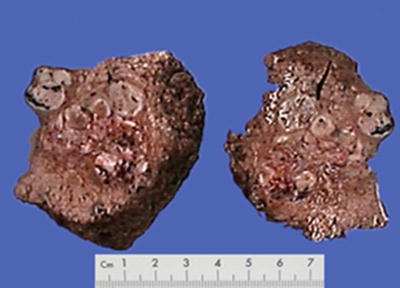
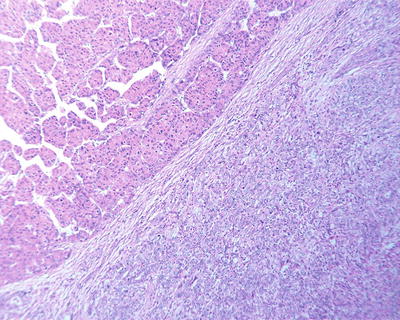

Fig. 7.134
Liver cancer, showing multiple-nodule type, showing multiple ICC and HCC nodules

Fig. 7.135
Liver cancer, collision type, showing completed fibrous capsule between HCC area (upper) and ICC area (below)
7.1.4.7 Treatment and Prognosis
Overall, cHCC-CC is a type of highly invasive tumors, and its long-term prognosis is poor; thus, surgical resection should be the first therapeutic option which is the principle of the treatment. The data provided by Shanghai Zhongshan Hospital (2012) showed that of the 103 cases of surgical resection cHCC-CC , the cumulative recurrence rates after 1 year, 3 years, and 5 years were 59.3, 85.1, and 98.2%, respectively, and overall survival rates after 1 year, 3 years, and 5 years were 73.9, 41.4, and 36.4%, respectively, suggesting the intermediate biological characteristics and surgical prognosis of cHCC-CC were between those of HCC and ICC [51]. The low long-term survival rate was related to the high recurrence rate (> 50%) after liver transplantation. Park et al. (2013) reported that cases of cHCC-CC had a high recurrence rate 1 year after liver transplantation [52]. Groeschl et al. (2013) provided the statistics of data from the database of “Surveillance, Epidemiology and End Results (SEER)” in the United States, showing that the survival rates 3 years after surgical resection of HCC and cHCC-CC were similar (55%:46%, P = 0.4), but the survival rate 3 years after liver transplantation in HCC patients was significantly higher than that in cHCC-CC patients (78%:48%, P = 0.01) [53]. Statistics on the data from SEER database was demonstrated by Garancini et al. (2014) showing that the overall survival rate 5 years after surgical resection and the disease-free survival rate reached 41.1 and 52.8%, respectively, suggesting that SEER stage and tumor size of 5–10 cm were independent prognostic factors which had an effect on the 5-year survival rate in these patients [41].
7.1.5 Dual-Phenotype HCC
We have noticed in pathological practice that a typical HCC in morphology can express markers of both the liver cell line (e.g., Hep Par-1, CK18, pCEA, etc.) and bile duct cell line (e.g., CK7, CK19, MUC-1, etc.), different from the cHCC-CC containing both HCC and CC components, and we named it as dual-phenotype HCC (DPHCC) [54]. Clearly, morphopathologically, DPHCC is not completely the same as cHCC-CC .
Based on the analysis on the characteristics of immunohistochemical phenotype, DPHCC accounts for about 5% of HCC. In clinical practice, DPHCC’s performance is similar to that of HCC, including a history of HBV or HCV infection, as well as chronic hepatitis and cirrhosis background, and elevation of both serum AFP and CA19-9 can be found in some cases. Histologically, DPHCC represents typical HCC features, such as polygon-shaped cancer cells, trabecular arrangement, sinuses, and pseudoglandular structure. In immunohistochemistry, the carcinoma cells, which express markers of liver cells with canalicular staining pattern for CD10 and pCEA, show the phenotypic characteristics of immunohistochemical proteins in CC (Fig. 7.136). Interestingly, the MVI found in DPHCC tissue maintained the dual phenotype, suggesting it is composed by cell populations with high proliferation and invasion activities (Fig. 7.137). According to our diagnostic criteria, the number of dual-phenotype positive cancer cells should be more than 15%, and they often show high expression of stem cell markers, such as EpCAM. DPHCC showed stronger invasiveness in biological behavior, and the incidence of satellite nodules and MVI is higher than that of common HCC. In the aspect of prognosis, the total survival rate and recurrence rate in patients with DPHCC are significantly poorer than those of the common HCC [54]. And our experimental studies showed that inhibition or upregulation of the expression of CK19 mRNA in HCC cells can markedly affect the proliferation and invasion of cancer cells. We hypothesized that DPHCC is a unique molecular subtype of HCC, originating from hepatic progenitor cells in the liver and maintaining a bidirectional differentiation potential, and it has a higher malignancy due to its dual biological behavior of HCC and ICC (Fig. 7.138). Govaere et al. (2013) also reported a lower invasive activity in the CK19 knockout HCC cell strain, as well as decreased drug resistance to 5-FU and sorafenib, suggesting the multiple drug resistance of DPHCC in clinical treatment [55]. Therefore, the routine pathological diagnosis should contain careful classification of special subtypes of HCC including DPHCC, to provide elaborate pathological information for clinical development of individualized diagnosis and treatment strategies.
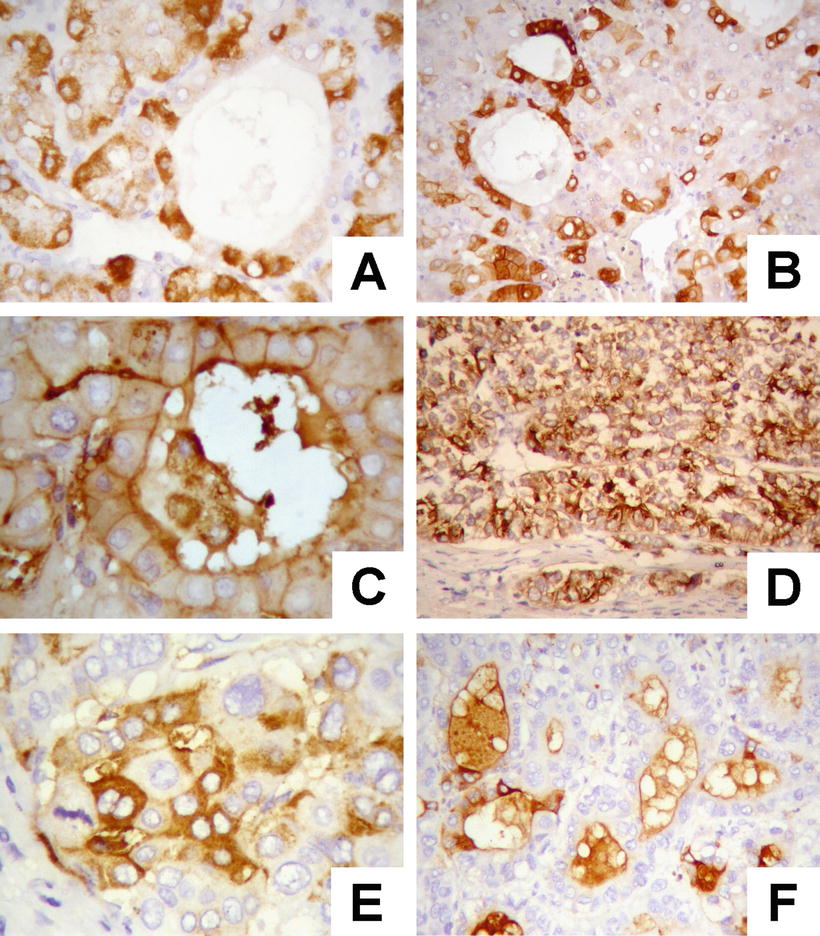
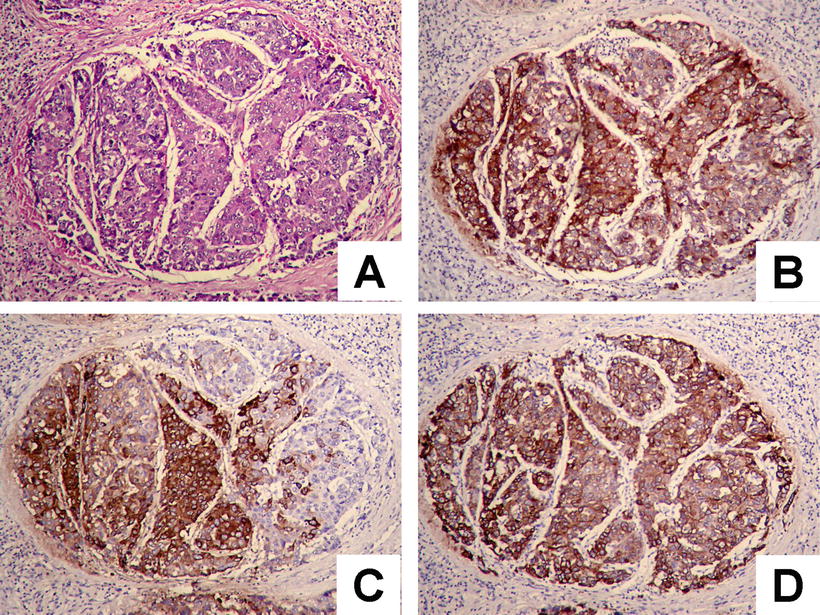
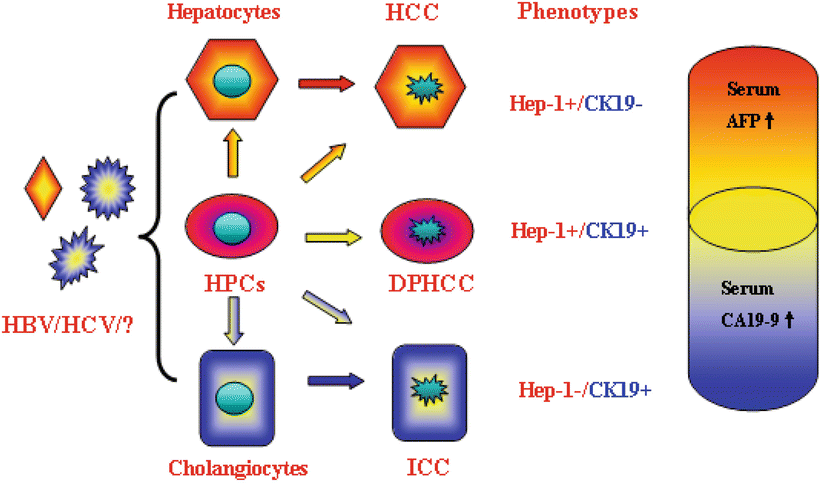

Fig. 7.136
Immunohistochemical staining of sequential series of tissue sections for DPHCC, (a) cytoplasmic stained for Hep Par-1; (b) cytoplasmic stained for CK19; (c) a bile canalicular stained for pCEA; (d) cytoplasmic stained for MUC-1; (e) cytoplasmic stained for CA19-9; (f) cytoplasmic stained for CA19-9

Fig. 7.137
Immunohistochemical staining of sequential series of tissue sections for DPHCC, (a) HE-stained section of MVI ; (b) pCEA-positive immunostaining of the same specimen as A; (c) Hep Par-1-positive immunostaining of the same specimen as A; (d) CK19-positive immunostaining of the same specimen as A

Fig. 7.138
Schematic diagram of the mechanism of DPHCC. HBV/HCV or other pathogenic factors may lead to the malignant transformation of hepatocytes, hepatic progenitor cells (HPCs), and cholangiocytes into HCC, DPHCC, and ICC , respectively. One hallmark of DPHCC cells is a bidirectional differentiation and can open both liver cell genes (e.g., Hep Par-1) and bile duct cell genes (e.g., CK19), and both serum AFP and CA19-9 contents may simultaneously increase (Reprint with permission from Cancer letter, 2015, Vol 368 (1), 14–19)
7.2 Malignant Bile Duct Tumors
Wen-Ming Cong3
(3)
Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University,, Shanghai, China
7.2.1 Intrahepatic Cholangiocarcinoma
7.2.1.1 Incidence
According to its anatomical origin, cholangiocarcinoma (CCA) can be divided into intrahepatic type (ICC ), perihilar type (pCCA), and distal type (dCCA), accounting for 5–10%, 60–70%, and 20–30%, respectively. These three types of cholangiocarcinomas are all independent lesions and are different from each other in epidemiology, pathological biology, clinical features, therapeutic strategies, and prognosis. The International Liver Cancer Association (ILCA) suggested that the names of Klatskin tumor and extrahepatic cholangiocarcinoma should be deprecated [56]. The term of cholangiocarcinoma usually refers to the malignant tumors of extrahepatic bile ducts, including two major types, namely, hilar cholangiocarcinoma and distal cholangiocarcinoma, while ICC refers to malignant tumors derived from intrahepatic biliary tree cells at all levels, including second-degree bile ducts in the liver adjacent to the hepatic portal, segmental bile ducts, and peripheral glands of the ducts (perihilar ICC ), or malignant tumors in the perihepatic regions derived from the epithelium of the subsegmental bile canaliculus (septal bile duct, interlobular biliary canals, and cholangiole) (peripheral ICC ) [57].
According to statistics, ICC accounted for 13% of all the tumor-related deaths of 7,600,000 per year in the world. The incidence rates of ICC in different countries vary greatly, and the annual incidence rate is in 113/100,000 in Thailand; 8.75/100,000 in Gwangju, South Korea; 7.55/100,000 in Shanghai, China; 3.4/100,000 in Osaka, Japan; 1.45/100,000 in Singapore; and 2.1/100,000 on average in Western countries including 1.67/100,000 in the United States [56]. Among 40,656 cases of hepatobiliary tumors diagnosed in the Department of Pathology of EHBH, Second Military Medical University during the 30-year period, malignant hepatic tumors accounted for 80%, and the top two tumors are HCC (86%) and ICC (8%), respectively.
7.2.1.2 Etiology and Pathogenesis
There are many known pathogenic factors closely related to the genesis of ICC , many of which are similar to those of HCC, including intrahepatic bile duct stones, chronic cholangitis, HBV/HCV infection, primary sclerosing cholangitis, chronic ulcerative colitis, parasite infection, bile duct malformation (common bile duct cyst and Caroli disease), cirrhosis, congenital hepatic fibrosis, fatty liver, diabetes, obesity, smoking, alcohol abuse, and environmental factors. The total lifetime prevalence rate of choledochal cyst is 5–30%, and the International Agency for Research on Cancer (IARC) listed Clonorchis sinensis as a class 1 carcinogen in 2009.
In recent years, the relationship between HBV/HCV infection and ICC has attracted increasing attention. The rate of positive serum HCV in ICC patients in Japan reached 36%, and the risk of developing ICC in patients with HCV-induced hepatic cirrhosis is 1000 times greater than in the general population [58]. Our recent statistics of the ICC cases with surgical resection in the EHBH during a period of nearly 30 years showed that the rates of HBV and HCV infection (confirmed via serum and/or immunohistochemical examination) were 47.44% and 9.64%, respectively. Litianyu et al. (2008) found HCV core gene-coding protein (HCVc) can induce transition of the cholangiocarcinoma cells from epithelial to mesenchymal phenotype and significantly promote their motility, invasion, and metastasis. Palmer et al. (2012) conducted a meta-analysis aiming at Western countries showed that the odds ratio (OR, 95% CI ICC ) defined risk factors for ICC include liver cirrhosis (22.92, 18.24–28.7), hepatitis type B (5.10, 2.91–8.95), hepatitis type C (4.84, 2.41–9.71), alcohol abuse (2.81,1.52–5.21), type 2 diabetes mellitus (1.89, 1.74–2.07), obesity (1.56,1.26–1.94), and smoking (1.31,0.95– 1.82) [59]. Chinese scholars (2012) reported that the ratio risk (RR, 95% confidence interval) of ICC in hepatitis type B patients was 3.42 (2.46:43.74) [60]. Razumilava et al. (2013) reported that the annual risk of developing cholangiocarcinoma for patients with primary sclerosing cholangitis in Western countries was 0.5–1.5%, and its lifetime incidence rate was 5–10% [61].
From the perspective of development in the study of molecular mechanisms of ICC , technological strategy in many studies on HCC can be applied to the research on ICC , and molecular variation of ICC and HCC is similar, such as the same genomic features and markers in ICC and HCC with poor prognosis, but they also have their own unique characteristics. The author (2001) studied 22 cases of ICC and found the rates of loss of heterozygosity/mutation in four kinds of tumor suppressor genes in ICC tissues were APC (68.8%), DCC (46.2%), OGG1 (41.7%), and p53 (37.5%), respectively, compared with those in HCC: p53 (87.5%), APC (58.8%), OGG1 (50%), and DCC (25%), showing the difference in the type and frequency of genetic mutations, suggesting the synergy of multigenetic mutations played an important role in the multistage development of ICC , and different molecular mechanisms and carcinogenesis pathways may be involved in the genesis of ICC and HCC, respectively [62].
According to the current understanding, common mutations in cholangiocarcinomas include KRAS (5–54%), TP53 (44%), SMAD4 (17%), and isocitrate dehydrogenases 1 and 2 (IDH1/2, 10–23%), as well as BRAF, NRAS, PI3K, EGFR, MET, PIK3CA (<5%), etc. And common methylated genes include p16INK4A (18–83%), suppressor of cytokine signaling-3 (SOCS-3.88%), RAS-associated domain family 1A gene (RASSF1A, 49%), and p14ARF (25%). Cell membrane receptors of therapeutic significance in molecular target therapies include ERBB2, EGFR, VEGFR, gp130/IL6-R, etc., and downstream signal transduction pathways of therapeutic significance in molecular target therapies include KRAS/MAPK, PI3K-AKT-mTOR, IL-6/STAT, COX-2/PGE2, etc., among which RAS-RAF-MEK-ERK signaling axis stimulates cell proliferation, and PI3K-AKT-mTOR signaling axis promotes cell survival [56, 63, 64].
Researchers have shown that interleukin-6 (IL-6) is one of the key cytokines involved in the carcinogenesis of bile duct epithelial cells and its main functions are as follows:
- ①
It can stimulate the bile duct epithelium and cancer cells to release IL-6 by autocrine or paracrine secretion in situations such as chronic inflammation, cholestasis, and tumor microenvironments, inducing high expressions of inducible nitric oxide synthase (iNOS), leading to high expression of reactive nitric oxide (RNOS), resulting in gene mutation and DNA strand breaks in cells.
- ②
Methylation silencing of SOCS-3 gene can be detected in both cholangiocarcinoma tissues and cells, which can lead to the absence of negative feedback regulation for IL-6 signal transduction pathways, resulting in increased expression of inflammatory molecules and upregulation of Mcl-1 gene which encodes the protein of antiapoptotic Bcl-2, and this can cause resistance to cytotoxic therapies in cholangiocarcinoma.
- ③
By enhancing the activity of telomerase, inhibiting the shortening of telomere, the cholangiocarcinoma cells can escape senescence.
- ④
IL-6 can affect the methylation of the promoter regions by regulating the activity of DNA methyltransferase, and overexpression of IL-6 can lead to the methylation of the promoters of a set of genes, such as the methylation of epidermal growth factor receptor (EGFR).
- ⑤
IL-6 receptor subunits, gp130 and gp130, can be highly expressed by the cholangiocarcinoma cells, which can be combined with IL-6 activating the downstream signal transduction pathways, such as JAK/STAT, PI3K/Akt and MAPK, resulting in the ability of long-term survival and continuous proliferation in cholangiocarcinoma cells through a series of cascase involving key molecular targets [56, 65, 66].
From the perspective of histogenesis, it was once believed that the ICC could be originated in the bile duct epithelial cells, periductal glandular epithelial cells, and hepatic progenitor cells. But with the application of advanced molecular biological methods, including the recent cell fate tracking technology, new discoveries will change the traditional understanding and theories. Fan et al. (2012) found that under the combined effects of Notch and AKT signal transduction pathways, the well-differentiated mature mouse hepatocytes can also develop ICC via transdifferentiation into bile duct epithelium [67], which helps understand the pathogenesis of ICC in patients with HBV/HCV hepatitis. Notch signaling pathway plays a key role in the development regulation of intrahepatic bile ducts and is closely related to activation of hepatic progenitor cells. Recent studies have shown that mice with expression of Notch-1 can develop ICC [68]. Andersen et al. (2012) found that cholangiocarcinoma with KRAS mutation has drug resistance to tyrosine kinase inhibitor therapy [69]. Oishi et al. (2012) suggested that miR-200 participated in the epithelial-mesenchymal transition (EMT) of ICC , directly targeting neural cell adhesion molecule 1 (NCAM1), and the expression of both molecules is associated with the prognosis, which may be the molecular markers of stem cell-like ICC with high invasion and the potential therapeutic targets [70]. Karakatsanis et al. (2013) found that miR-21 and miR-221 in HCC tissues were significantly upregulated and were related to tumor staging and the prognosis [71]. In addition, the relationship between ICC and gene polymorphism of enzyme systems, which are related to DNA methylation, DNA damage/repair and biliary toxins clearance, has also been highly valued [71]. In a word, although the pathogenesis of ICC is not entirely clear, several important progresses have been made. And a summary of the main knowledge in the pathogenesis of ICC was made in Fig. 7.139, which is also the basis and focus of further study on molecular mechanism of ICC .
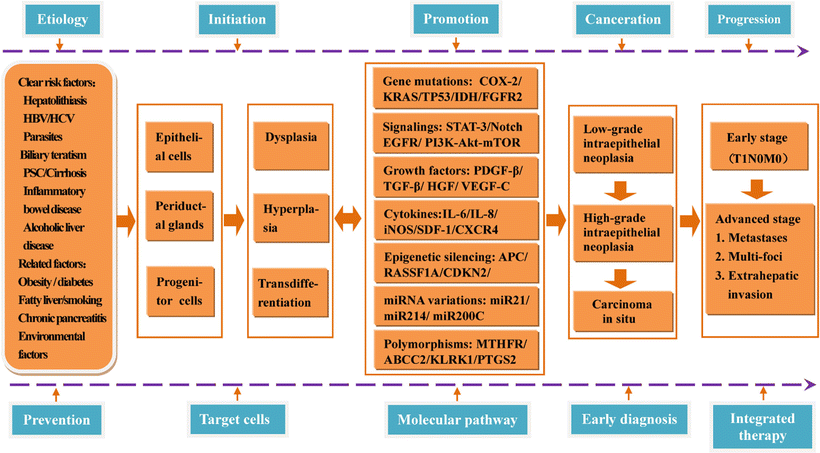

Fig. 7.139
Possible pathogenesis of multiple stages of ICC development
7.2.1.3 Pathological Staging of ICC
Currently, it has been recognized that ICC and HCC are different in many aspects, including pathogenic mechanism, histogenesis, biological characteristics, molecular targets, and prognostic risk factors. Therefore, it is necessary to study the clinical pathologic staging system with the characteristics of ICC . The following five staging systems for ICC thoroughly reflect the current understanding of clinical pathological parameters which affects the prognosis of ICC . In the future, it should be considered to add in the indexes which can reflect the mole biological characteristics of HCC.
- 1.
AJCC/UICCTNM-ICC (seventh edition) (2009). According to the number of tumors, invasion of the adjacent tissues, vascular invasion, lymphatic metastasis, and distant metastasis, as well as other parameters of ICC , the TNM staging of ICC (including cHCC-CC ) (Table 7.7) was proposed. The specifications of several terms are shown as follows: multinodular tumors including multiple primary tumors and satellite nodules, vascular invasion including gross appearance of invasion of portal vein, venous thrombosis and MVI , regional lymphatic metastasis including hepatic hilar lymph nodes with drainage through the right lobe of the liver and hepatic portal areas, peri-duodenal lymph nodes and peri-pancreatic lymph nodes, as well as the lymph nodes of ligamentum hepatogastricum, and distal lymphatic metastasis referring to the involvement of lymph nodes in the abdominal cavity, around the abdominal aorta and inferior vena cava without any definition of the number of lymph nodes, T4 classification simply based on the gross type of ICC . However, whether the periductal infiltrating type is suitable for use as an independent grading index of advanced ICC , and poor prognosis needs to be confirmed by more evidence-based information [72]. The 5-year survival rate of ICC in TNM I stage with R0 resection can reach 40%.
Table 7.7
The seventh AJCC staging system for ICC
TNM staging
Characteristics
T1
Single tumor without vascular invasiona
T2a
Single tumor with vascular invasiona
T2b
Multiple tumors, with or without vascular invasiona
T3
Tumor(s) perforating the visceral peritoneum or involving the local extrahepatic structures by direct invasion
T4
Tumor with periductal invasionb
N0
No regional lymph node metastases
N1
Regional lymph node metastasesc
M0
No distant metastases
M1
Distant metastases
Staging
Stage I
T1 N0 M0
Stage II
T2 N0 M0
Stage III
T3 N0 M0
Stage IVA
T4 N0 M0, Any T, N1 M0
Stage IVB
Any T, Any N, M1
- 2.
Nathan’s pathological staging for ICC (2009): Aiming at the problem of insufficient stratification of T stages in TNM staging system, a simplified T staging including core indexes was proposed, including vascular invasion, number of the tumors, and extrahepatic metastasis. Although the rate of lymphatic metastasis in ICC cases reached 32% which was an independent predictor for poor prognosis in ICC , it is not included in this index system, since lymph node dissection has not yet been included into routine operation of ICC (Table 7.8) [73].
Table 7.8
Nathan’s pathological staging for ICC
Staging
Index
Microvascular invasion
Multiple tumors
Diameter of the tumors
sT1
Without
Without
All
sT2
With
Without
All
Without
With
All
With
With
All
sT3
Invasion of extrahepatic tissuesa
Staging
Stage I
sT1, N0, M0
Stage II
sT2, N0, M0
Stage III
sT3, N0, M0, or N1 M0 (any sT)
Stage IV
M1 (any sT, any N)
- 3.
Liver Cancer Study Group of Japan (LCSGJ) (2003) staging for ICC LCSGJ divided ICC into three gross types, including mass-forming (MF), periductal-infiltrating (PI), and intraductal-growing (ID) types, and in view of rare cases of the latter two types of ICC , the TNM staging proposed by LCSGJ is only for mass-forming-type ICC (Table 7.9) [74].
Table 7.9
TNM staging for ICC by Liver Cancer Study Group of Japan
Staging
T
N
M
Stage I
T1
N0
M0
Stage II
T2
N0
M0
Stage III
T3
N0
M0
Stage IVA
T4
N0
M0
Or all T
N1
M0
Stage IVB
Any T
Any N
M1
- 4.
Fudan’s prognostic scores for ICC (2013): A 5-score staging system consisting of five parameters with five points for each of them, including serum alkaline phosphatase (ALP), CA19-9, number of tumors, maximum diameter of the tumors, and tumor types, screened from 244 patients. The score of 0, 1, 2–3, and 4–5 indicates low, moderate, high, and very high risk, respectively (Table 7.10), and the 5-year survival rates of patients with the above corresponding scores are 48.6%, 25.6%, 10.3%, and 0, respectively [75].
Table 7.10
Fudan’s prognostic scores for ICC
Factors
Score
Serum ALP (U/l)
≤147.0
0
>147.0
1
Serum CA19-9 (μg/l)
≤37.0
0
>37.0
1
Tumor number
Single
0
Multiple (≥2个)
1
Maximum tumor diameter (cm)
<10
0
≥10
1
Tumor boundary
Clear
0
Unclear
1
Risk grouping
End index
Risk ratio (95% confidence interval)
P
Low risk
0
1
0.01
Moderate risk
1
2.001 (1.180–3.394)
<0.001
High risk
2
3.719 (2.179–6.347)
<0.001
3
5.456 (3.162–9.413)
<0.001
Very high risk
4
12.255 (6.344–3.556)
<0.001
5
27.519 (9.944–79.152)
<0.001
- 5.
EHBH’s prognosis assessment for ICC (2013): In EHBH, based on the pathological analysis of 367 cases of surgically resected ICC , a nomograph was proposed consisting seven parameters, including serum CEA, CA19-9, vascular invasion, lymphatic metastasis, direct invasion and local metastasis, number of tumorous nodules, and diameter of the tumors. The value of each parameter can be calculated through their conversion into counts listed on the first line, and the added numerical value of a patient should be marked on the chart of total index value. And it can be used to evaluate the prognosis of a patient conveniently and intuitively by comparison of the added numerical value and the 3-year and 5-year survival rates (Table 7.11) [76].
Table 7.11
Nomograph of prognosis assessment of ICC in EHBH
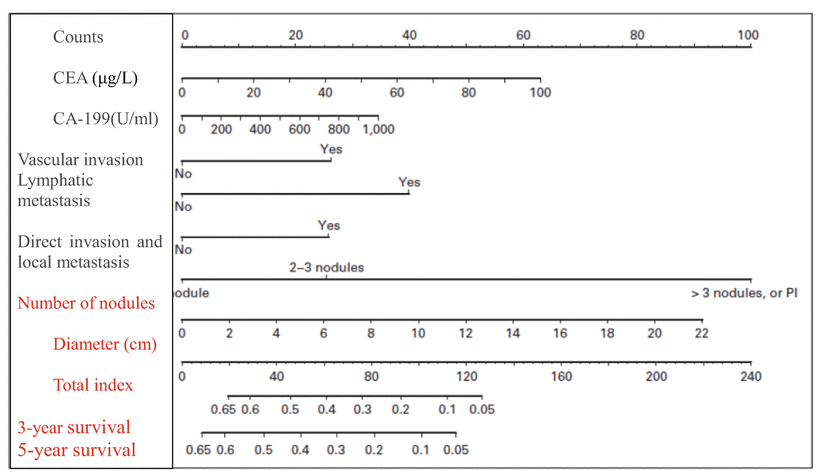
According to a study on postsurgical information of 67 cases of ICC in EHBH of Second Military Medical University, it was shown that 22 cases (32.8%) had lymphatic metastasis and the metastasis rate of primary tumors in the left lobe of the liver was 47%, which was significantly higher than that of the right lobe of the liver (18.2%). In addition, skipping lymphatic metastasis has also been observed in these cases, of which the metastasis rate of lymph nodes on ligamentum hepatoduodenale was the highest, indicating the lymph nodes on ligamentum hepatoduodenale can be regarded as the sentinel lymph nodes in ICC [77].
7.2.1.4 Clinical Features
Of all the 2602 cases of ICC diagnosed in the Department of Pathology of EHBH during the 30 years, the patients were 53.35 years old on average with the male to female ratio of 2.22:1. The clinical features of peripheral ICC are not specific, and obvious symptoms were found in early stage, while patients with progression ICC may exhibit clinical manifestations similar to those of HCC, including hepatomegaly, pain in the hepatic zone, decreased apatite, weight loss, fatigue, abdominal distension, and abdominal mass. Biliary obstructive jaundice was rare. The diagnostic sensitivity and specificity of significantly higher serum CA19-9 levels (>100 U/ml) were 89% and 86%, respectively. The sensitivity of CEA was 30%, and the sensitivity of intraductal ultrasound was 89–95%. About 25% of ICC patients had elevated serum AFP levels to different degrees, especially in patients with chronic viral hepatitis and cirrhosis patients. Ultrasound type B shows ICC as intrahepatic hypoechoic mass with smooth and irregularly thickened margins. Dynamic CT images display gradual enhancement or delayed enhancement of ICC lesions [78, 79].
7.2.1.5 Gross Features
ICC lesions are often found in the left lobe of the liver, with a diameter of 2–15 cm, and the sections are usually gray white, homogeneous, and tender in texture, with less hemorrhage and necrosis. Most cases have no fibrous capsule, showing an invasive border, and the liver tissues often contain cholestasis and little cirrhosis.
The gross morphology of ICC is similar to that of HCC with its own characteristics, which can be roughly divided into the following nine gross types:
- 1.
Mass-forming type: It is the most common type (> 85%), forming masses or nodules in the liver parenchyma, and the tumors are gray white, dense, and tender in texture because of fibroconnective tissues, often with no fibrous capsule (Fig. 7.140), prone to intrahepatic metastasis.
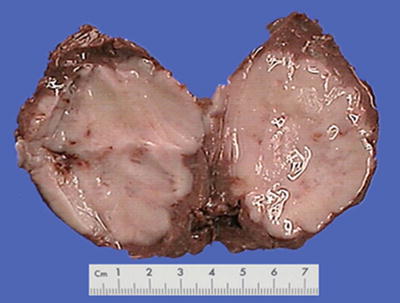
Fig. 7.140
ICC , mass-forming type, gray-white tumor with tough and dense connective tissue
- 2.
Periductal-infiltrating type: The tumors do not form masses but expand to both sides along the wall of bile ducts, resulting in thickened wall of the involved bile ducts, luminal stenosis, and dilatation of peripheral bile ducts (Fig. 7.141), and tumors often invade the liver parenchyma along the portal vein or disseminate along the interlobular biliary canals in the portal area leading to portal lymphatic metastasis in the liver.
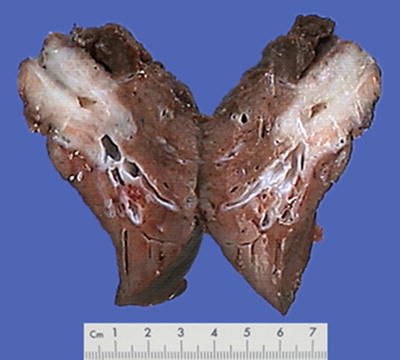
Fig. 7.141
ICC , periductal-infiltrating type, tumor growing along the bile duct
- 3.
Intraductal growth type: Polypoid or papillary tumors grow and protrude into the lumen of bile ducts, with or without invasion of the bile duct wall (Fig. 7.142).
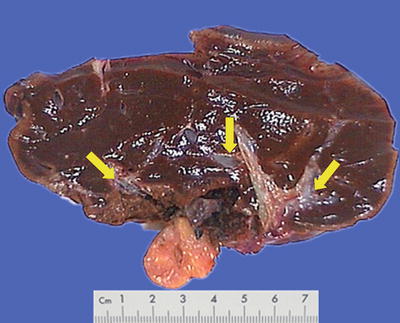
Fig. 7.142
ICC , intraductal growth type, bile duct filled with gray and white jelly-like tumor tissue
- 4.
Superficial spreading type: The cancer cells are arranged in a micropapillary structure, which only invade and grow in the epithelial layer of the bile duct with no invasion of the bile duct wall (Fig. 7.143).
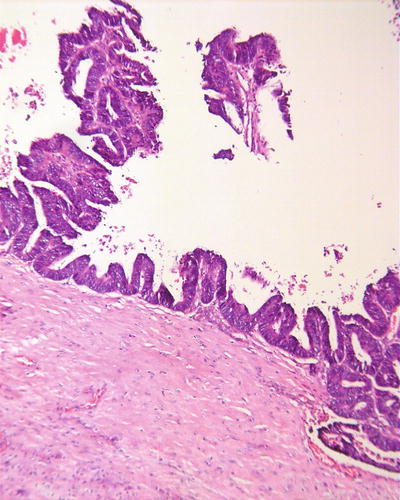
Fig. 7.143
ICC , superficial spreading type, tumor infiltrating through the mucous layer
- 5.
Multinodular type: The tumor is composed by a number of tumor nodules which can grow in confluent multinodular pattern (Fig. 7.144). And stones can be found in the bile ducts in cases with secondary carcinoma derived from the bile duct stones (Fig. 7.145).
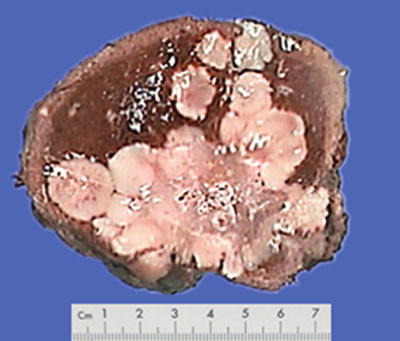
Fig. 7.144
ICC , multinodular type, nodules enlarge and become confluent
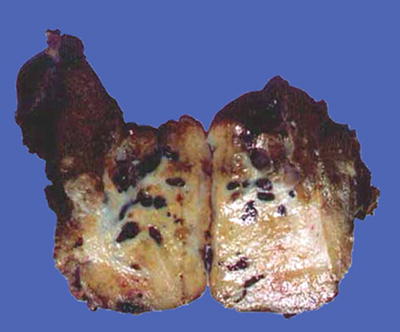
Fig. 7.145
ICC , mass-forming type, showing calculus of the bile duct
- 6.
Diffuse type: The tumor shows multiple nodules in diffuse growth and distribution in the liver (Fig. 7.146).
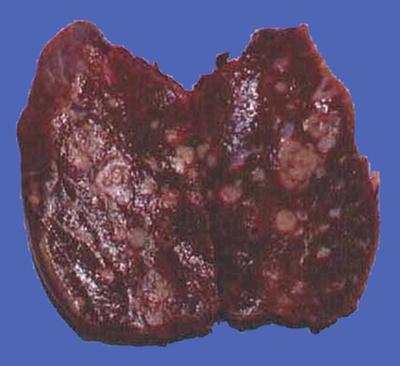
Fig. 7.146
ICC , diffuse type, tumor nodules scattered throughout the liver parenchyma
- 7.
Massive type: The diameter of the tumor reaches >10 cm (Fig. 7.147).
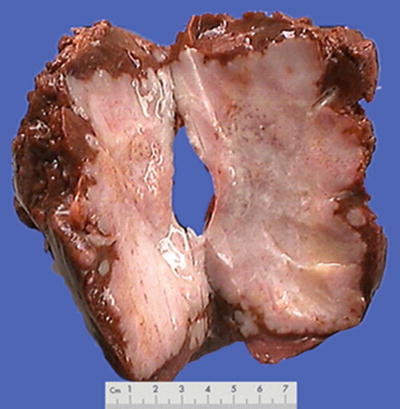
Fig. 7.147
ICC , massive type, a huge mass >10 cm with the boundary not well defined
- 8.
Small carcinoma type: The diameter of the tumor is ≤3 cm (Fig. 7.148).
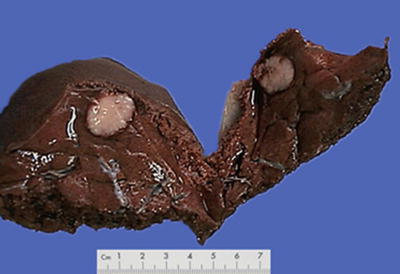
Fig. 7.148
ICC , small ICC , a small nodule <3 cm with a clear boundary
- 9.
Mixed-type combinations of the above types: It has been pointed out that ICC ≥5 cm is an important index to predict MVI and poor differentiation of ICC cells [80].
7.2.1.6 Microscopic Features
ICC lesions are mostly moderately or well-differentiated tubular adenocarcinoma, and the size of tumorous glands is associated with the grade of the branches in the biliary tree. In general, central-type ICC mainly involves grade 1–3 major branches of the bile ducts, and the large glands in the tumor consisting of tall columnar cells are multicapillary and grow invasively along the bile duct wall (Fig. 7.149). Gradual transition between the cancer tissue and intraepithelial neoplasia of epithelium of the bile ducts in different degrees can be found in some cases (Fig. 7.150). Intraductal-type ICC is characterized by inward growth of the tumor in the bile ducts, with tumorous glands filling the lumen, but no obvious invasion of bile duct wall is observed (Fig. 7.151). Peripheral-type ICC mainly involves the septal bile duct and interlobular bile ducts, and the cancer cells are cuboidal, with tumorous glands sized between the major bile ducts and bile canaliculi, while different diameters of the bile ducts can be found on the same slice (Fig. 7.152). The fibrous interstitial tissue varies according to different density of the glands (Fig. 7.153). Cholangiocarcinoma of bile canaliculi (cholangiocellular carcinoma) originating in the canals of Hering is a special subtype of ICC , with tumorous glands composed of small cuboidal cells and small and regular lumens, which may be similar to the proliferated small bile ducts, and it is rich in fibrous stroma (Fig. 7.154). It is specifically discussed in Part 4 of this section.