Histological subtype
AFP
HCG
Primitive germ cell tumours
Dysgerminoma
−
±
Yolk sac tumour
++
−
Embryonal carcinoma
±
±
Polyembryona
±
+
Non-gestational choriocarcinoma
−
++
Mixed germ cell tumour
±
±
Biphasic or triphasic teratoma
Immature teratoma
+
−
Mature teratoma
−
−
Monodermal teratoma and somatic-type tumours
In most large case series, dysgerminoma represents approximately 15–25 % of cases, teratoma 12–20 %, yolk sac tumours 25–30 % and mixed germ cell tumours 30–35 %. The importance of correct histological diagnosis is underpinned by the therapeutic implications for the patient.
Some authorities have recommended a two-tier grading system for immature teratomas because of inter- and intraobserver inconsistency with a three-grade system. Additionally, the criteria to establish malignant behaviour in immature teratomas vary among reports and institutions based on the amounts of immature neural and/or nonneural elements and yolk sac elements.
As these tumours are chemosensitive and fertility-preserving surgery is utilised, the recent ESMO guidelines [6] stipulate that all such tumours should be considered for second review by an expert histopathologist. Indeed in some centres, this central review of pathology is mandatory. In the UK there has now been a move to centralise the care of MOGCT to mirror the successful approach used used in Gestational Trophoblastic Disease (GTD) [11].
Tumour markers play an important role in the diagnosis and management of germ cell tumours. The serum alpha-fetoprotein (AFP), lactate dehydrogenase (LDH) and serum human chorionic gonadotropin (hCG) should be measured at baseline in all patients. AFP is secreted by yolk sac tumour elements and may be secreted by embryonal carcinoma and immature teratomas. The pregnancy hormone, hCG, is secreted by choriocarcinoma elements and can be produced in a small subset of dysgerminomas at lower levels [8]. Mixed germ cell tumours may secrete a combination of AFP and hCG, and approximately 16 % may be negative for all tumour markers. Dysgerminoma never secretes AFP, and about 10–20 % produces hCG [12, 13]. All MOGCT may be associated with an elevated lactate dehydrogenase level and CA125 both of which are non-specific for the disease but may nevertheless give an indication of response and relapse. In practical terms, the ability to regularly monitor tumour markers following any intervention, such as either surgery or chemotherapy, allows the clinician to have an early readout of response, resistance and relapse. For example, failure to normalise tumour markers after curative surgery in stage 1 disease would be an indicator for further treatment (stage 1 M disease). Furthermore, knowledge of the normal half-life of these tumour markers is helpful in this regard. If all the tumour has been removed, then for patients with normal renal and liver function, the half-life of hCG is 1–2 days and for AFP is about 6–7 days. If the markers are falling slower than this, then suspicion about residual disease should be high. If gonadal dysgenesis is suspected based on history and physical examination, a karyotype should be performed before surgery, if possible, to ensure both ovaries are removed if clinically indicated.
Prognostic Factors
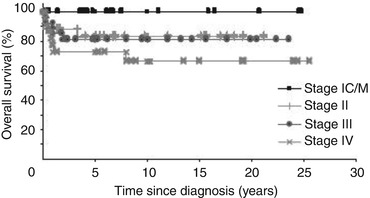
FIGO stage at presentation is the most critical indicator of prognosis. Evidence from many centres indicates that stage I (including 1a, 1c and 1 m) has an excellent prognosis of approaching 100 % survival rates. This cure rate progressively declines to approximately 80–90 % in stage II and stage III disease and around 60 % cure rates in stage IV disease (see Fig. 18.1) [14–16]. Other factors have been identified that can be independent prognostic indicators in univariate and multivariate analyses. Indeed, in those patients receiving chemotherapy (stages 1c/1 m through stage IV at diagnosis), the presence of elevated AFP and hCG at diagnosis was associated with a much poorer outcome when compared to those patients with both tumour markers being normal at baseline [16]. The role of age as an independent prognostic factor is more controversial with some studies suggesting this and others not [15, 17]. It is clear however that paediatric MOGCT may represent biologically different group diseases as recent studies have indicated significant differences in transcriptional signatures more suggestive of pluripotency compared to adult tumours [18]. Indeed outcomes in the paediatric population differ with seemingly higher survival when matched stage for stage with adults [19]. Residual disease after surgery and histological subtype (dysgerminoma vs non-dysgerminoma) have also been implicated as prognostic factors in retrospective case series [20].
Management of Early Disease
Surgery is the mainstay of management of early MOGCT. In other gynaecologic malignancies, the principles of cancer surgery dictate maximal clearance with a bilateral salpingo-oophorectomy (BSO), total abdominal hysterectomy (TAH) and peritoneal exploration. However, in MOGCT, fertility-preserving surgery should be utilised [21]. For most patients with early stage disease (stage 1A/1B), a unilateral salpingo-oophorectomy (USO), peritoneal washings and careful exploration of the abdomen are appropriate. Routine biopsy of the remaining ovary should be avoided unless there is clinical suspicion of bilateral disease. This last point is important as most MOGCTs are unilateral, with dysgerminomas having the greatest propensity for contralateral disease in the other ovary, and this is said to be seen in up to 15 % of these tumours although in our experience, it seems less than this [22]. Avoiding the contralateral ovarian biopsy potentially helps future fertility by preventing scar formation over the ovary surface.
The management of early MOGCTs that have been pathologically staged as 1A/1B (and as such are confined to the ovary with no capsular rupture) has been controversial with some centres advocating the use of adjuvant chemotherapy in addition to fertility-preserving surgery, particularly in non-dysgerminomatous histologies (grade II–III immature teratoma, mixed GCT and yolk sac tumours) [23]. Studies by Williams et al. [24], Gershenson [25] and Dimopoulous et al. [26] have demonstrated excellent survival outcomes in completely resected MOGCT treated with adjuvant bleomycin, etoposide and cisplatin (BEP), but these studies have to be interpreted with caution as they also include stage II and III diseases. There is consensus that stage 1A dysgerminoma should be managed with surgery alone with many studies showing survival of close to 100 % with a policy of surveillance after fertility-sparing surgery [27]. A policy of careful surveillance after surgery for stage 1A/1B disease of all histological subtypes has been adopted by Charing Cross and Mount Vernon hospitals with long-term survival in excess of 95 % in these patients, reserving chemotherapy only for those patients that relapse [27]. This approach has subsequently been adopted, and its efficacy validated in other centres [23] as well as in a prospective study in the paediatric and adolescent population [28]. In keeping with this, the recent ESMO guidelines assert that chemotherapy is not mandated in the adjuvant setting in non-dysgerminomatous early stage MOGCT and can be reserved for documented relapse after a policy of close surveillance.
The Management of Advanced Disease
Cytotoxic chemotherapy has altered the landscape of MOGCT prognosis. In the pre-chemotherapy era, even patients with early stage disease would be at significant risk of relapse and death, and all patients with advanced disease would die from their disease. Early studies demonstrated some efficacy of VAC (vincristine, dactinomycin and cyclophosphamide) combination chemotherapy in patients with MOGCT [29], but most lessons have been learnt from the randomised studies undertaken in testicular germ cell tumours. As a result of this work, the regimes used in MOGCT have been optimised to allow maximal preservation of fertility, whilst decreasing risk of long-term complications from agents such as cyclophosphamide (in its propensity to cause acute leukaemia). Cisplatin-based regimens with PVB (cisplatin, vinblastine, bleomycin) [30] then most recently BEP chemotherapy (bleomycin, etoposide, cisplatin) have become the gold standard therapy for advanced or relapsed MOGCT. Studies using platinum-based combination regimens have demonstrated overall survival in all MOGCT patients requiring chemotherapy (both those with relapsed early stage disease following surveillance and advanced disease) of between 62 and 85 % (Table 18.2) [20, 29, 31–33]. Although BEP is the most commonly utilised regimen in this setting, with evidence demonstrating efficacy and overall survival of 82–90 % [14, 20, 31, 34] across all stages, the optimum regimen is still unclear due to the lack of a randomised evidence base in this cohort of patients with MOGCT. Studies in germ cell tumours have examined the efficacy of substituting carboplatin with cisplatin due its markedly different toxicity profile including less nausea, ototoxicity and renal toxicity. However, the data available does not demonstrate that two platinum agents are comparable, and there is a suggestion (in testicular GCT) that carboplatin outcomes may be inferior [35]. Most centres would therefore recommend using cisplatin-containing regimens in MOGCT unless there are specific contraindications that make safe delivery of cisplatin impossible.
Table 18.2
Studies of platinum-based chemotherapy in advanced MOGCT
Study | Patients (n) | Regimen | Overall survival | Includes stage 1A |
|---|---|---|---|---|
Williams et al. | 93 | BEP | 96 % | Yes |
Murugaescu et al. | 113 | BEP/POMB-ACE | 82 % | No |
Gershenson et al. | 26 | BEP | 96 % | Yes |
Dimopoulous et al. | 16 | BEP | 88 % | Yes |
Lai et al. | 93 | BEP | 97 % | Yes |
Billmire et al. | 131 | BEP | 97 % | Yes |
Zanetta et al. | 105 | BEP/PVB | 95 % | Yes |
The POMB-ACE regimen was introduced by Charing Cross Hospital to manage patients with high-risk-advanced testicular germ cell tumours. It was found to be a well-tolerated and effective method of introducing seven cytotoxic agents in a two-weekly alternating regimen to try and overcome drug resistance and maximise cure rates [36]. Indeed, in poor prognosis testicular GCTs, the cure rate with POMB/ACE is around 70 % which from nonrandomised data appears better than four cycles of BEP. In stage IV MOGCT, cure rates appear to be around 65 % with this regimen, but data are still limited, and there is a clear need to improve outcomes for this group of patients [16].
A current consensus would indicate that for relapsed stage I disease or for stage 1c and 1 m and completely resected stage II–III disease, three cycles of BEP chemotherapy would be standard treatment. In incompletely resected stage III or IV disease with a higher burden of tumour, including patients with very high tumour markers, a longer course of platinum-based chemotherapy is utilised, commonly four cycles of BEP over 12 weeks or 5–7 treatments with POMB-ACE chemotherapy over 10–14 weeks [6, 16]. However, there is a clear need to improve management for these advanced cases to improve overall survival results.
The value of aggressive surgery prior to chemotherapy in advanced disease is debatable. This is because residual microscopic disease grows back so fast that by the time chemotherapy is commenced post-operatively, much of the cytoreduction advantage of the surgery is lost. Moreover, with so much disease in the pelvis, fertility-preserving surgery becomes very difficult. Consequently, many centres now practise neoadjuvant chemotherapy for advanced MOGCT and reserve surgery for once the chemotherapy is completed. In some instances this will mean that only the affected ovary is left to remove, but in others, the disease will become more cystic and large residual masses will need to be excised. Leaving these masses is not sensible as one cannot be certain that there is no active cancer left, and over time they may continue to grow in up to 30 % of patients and in a small proportion may dedifferentiate back in to active cancer.
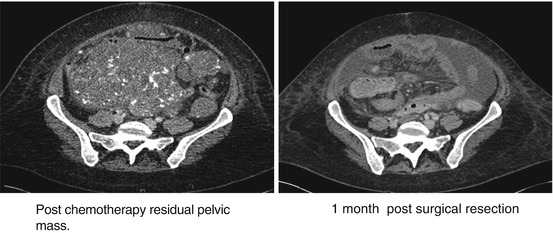
With chemotherapy, once the tumour markers have normalised, it may help to continue treatment for a further 4–6 weeks to help minimise the possibility of relapse. Re-imaging after treatment with an MRI pelvis, CT chest and abdomen all with contrast will help define what needs surgical resection. Various features of the clinical course may cause concern for the managing clinicians. Thus, in some patients the AFP will fail to return to normal or might start rising towards the end of chemotherapy which can reflect liver repair and production of AFP rather than progressing disease. In addition, some patients with cystic differentiation may develop mature teratoma-growing syndrome, so post-chemotherapy imaging may show enlarging masses that make the clinicians feel their treatment has failed. However, once this is resected, this will nearly always turn out to be mature teratoma which requires no further chemotherapy. It is essential that residual masses are removed. Small lesions of 1 cm or less that appear non-cystic can be observed with repeat imaging 2–3 months post-completion of chemotherapy. Figure 18.2 illustrates an example of residual cystic pelvic mass post-POMB-ACE chemotherapy and marker normalisation. This mass was resected and showed mature-differentiated cystic teratoma. The CT images show the appearances before and after surgery [6].