Free and total testosterone
Only 2% of plasma testosterone is free (unbound). Of the rest, 44% of testosterone is bound to a hepatic glycoprotein called sex hormone-binding globulin (SHBG), and 54% of testosterone is loosely bound to albumin. Almost all of the albumin-bound testosterone is available for tissue uptake. Therefore bio-available testosterone in plasma is the sum of free (2%) plus albumin-bound hormone (54%).
The serum SHBG levels may be increased and decreased by a number of factors (Box 19.1).
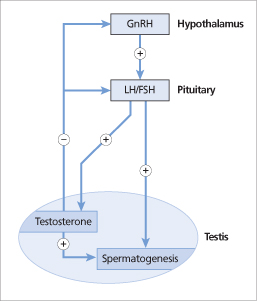
Figure 19.2 Pulsatile gonadotrophin-releasing hormone (GnRH) release from the hypothalamus stimulates luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release from the anterior pituitary. LH stimulates testosterone synthesis by the Leydig cells. Sperm are produced under stimulation by testosterone and FSH. LH secretion is inhibited by testosterone, which acts on the hypothalamus and directly on the anterior pituitary.
However, changes in the SHBG levels do not affect free androgen levels. This is because the hypothalamic-pituitary system responds to acute changes in the concentrations of bioavailable testosterone (caused by changes in SHBG levels) by altering testosterone synthesis.
Mechanism of action of testosterone
Testosterone is converted to active metabolites 5α-dihydrotestosterone (by 5α-reductase) and 17β-oestradiol (by aromatase). Testosterone and its metabolites bind to intracellular receptors, which in turn bind to specific DNA sequences (‘response elements’) and regulate the transcription of certain genes.
Physiological actions of testosterone
The physiological actions of testosterone are the result of the combined effects of testosterone itself plus its active metabolites. The major functions of androgens in males include:
- the regulation of gonadotrophin secretion from the hypothalamic–pituitary system
- the initiation and maintenance of spermatogenesis
- the formation of the male genital tract during embryogenesis
- the development of male secondary sexual characteristics and sexual potency at puberty, and their maintenance thereafter.
Male hypogonadism
Male hypogonadism is a syndrome of decreased testosterone production, sperm production or both.
Aetiology
Male hypogonadism may result from disease of the testes (primary hypogonadism) or disease of the pituitary or hypothalamus (secondary hypogonadism).
Known causes of hypogonadism are summarized in Box 19.2. However, many cases of hypogonadism remain unexplained (idiopathic).
Primary hypogonadism
In primary hypogonadism, reduced testosterone levels result in elevated gonadotrophin levels (due to a reduced negative feedback effect of testosterone on the hypothalamus and pituitary). Thus primary hypogonadism is also known as hypergonadotrophic hypogonadism. Primary hypogonadism may be congenital or acquired.
Congenital causes
Congenital primary hypogonadism may be due to Klinefelter’s syndrome, other chromosomal abnormalities or cryptorchidism.
Klinefelter’s syndrome is the most common congenital cause of primary hypogonadism and occurs in about 1 in 500–1000 live male births. It is caused by one or more extra X chromosomes in men, resulting in damaged seminiferous tubules and Leydig cells. The greater the number of extra X chromosomes, the greater the phenotypic consequences. The most common genotype is 47XXY. The 47XXY genotype results from non-disjunction of the sex chromosomes of either parent during meiotic division. 46XY/47XXY mosaicism probably results from non-disjunction during mitotic division after conception.
In addition to features of hypogonadism, patients may have:
- intellectual dysfunction and behavioural abnormalities that cause difficulty in social interactions
- a predisposition to develop chronic bronchitis, bronchiectasis and emphysema, germ cell tumours (e.g. involving the mediastinum), breast cancer (a 20 -fold increased risk), possibly non-Hodgkin’s lymphoma, varicose veins, leg ulcers and diabetes mellitus.
A large number of other chromosomal abnormalities have been reported that result in testicular hypofunction. The 46XY/X0 karyotype results in a syndrome characterized by short stature and features typical of Turner’s syndrome (see Chapter 21). Gonadectomy should be performed in a patient who has both a streak gonad and a dysgenetic testis, as the risk of gonadoblastoma is about 20%. Up to 20% of men with azoospermia or severe oligospermia have microdeletions in specific regions of the long arm of the Y chromosome.
Cryptorchidism refers to unilateral or bilateral (10%) undescended testes (in the abdominal cavity or in the inguinal canal) that cannot be manipulated manually into the scrotum by the age of 1 year. The risk of testicular cancer is increased (3–14-fold).
Acquired causes
Acquired primary hypogonadism may be due to infections (e.g. mumps orchitis), testicular trauma or torsion, chemotherapy, radiotherapy, autoimmune damage or chronic illnesses (e.g. chronic obstructive pulmonary disease, congestive cardiac failure, Crohn’s disease, coeliac disease, chronic liver disease, chronic kidney disease, chronic anaemia, rheumatoid arthritis, AIDS).
Secondary hypogonadism
Secondary (or hypogonadotrophic) hypogonadism is due to impaired secretion of hypothalamic GnRH or pituitary gonadotrophins. Secondary hypogonadism may be congenital or acquired.
Congenital GnRH deficiency
Congenital secondary hypogonadism may be associated with anosmia in Kallmann’s syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree