Resectability status
Arterial
Venous
Resectable
No arterial tumor contact
No tumor contact with the superior mesenteric vein (SMV) or portal vein (PV) or ≤180° contact without vein contour irregularity
Borderline resectable
Solid tumor contact with HA without extension to celiac axis or hepatic artery bifurcation allowing for safe and complete resection and reconstruction
Solid tumor contact with the SMA of ≤180°
Solid tumor contact with the CT of ≤180°
Solid tumor contact with the SMV or PV of >180° and contact of ≤180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal to the site of involvement allowing for safe and complete resection and vein reconstruction
Solid tumor contact with the inferior vena cava
Unresectable
Distant metastasis (including non-regional lymph node metastasis)
Solid tumor contact with SMA >180°
Solid tumor contact with the CT >180°
Solid tumor contact with the first jejunal SMA branch
Solid tumor contact with the CA and aortic involvement
Unreconstructible SMV/PV due to tumor involvement or occlusion (can be due to tumor or bland thrombus)
Contact with most proximal draining jejunal branch into SMV
Unreconstructible SMV/PV due to tumor involvement or occlusion (can be due to tumor or bland thrombus)
15.3 Neoadjuvant Therapy
Patients with borderline resectable PDAC can be considered as potential candidates to receive neoadjuvant radio-/chemotherapy [15]. However, the treatment scheme (gemcitabine vs. 5-FU-based protocols + radiation) is still being discussed conversely. New studies demonstrate that neoadjuvant treatment should be done with the combination of FOLFIRINOX and radiation since this combination showed promising short-term outcomes allowing more potential R0 resection [22, 23]. Tumor response like downsizing and development of fibrosis is reevaluated after 4–6 weeks.
To determine the correct approach in each patient, it is of great importance to preoperatively distinguish between an arterial and a venous involvement of the PDAC. In the case of a venous involvement, no neoadjuvant treatment is recommended, and surgical resection should be planned accordingly if technical options for resection are possible. Though, higher rates of intraoperative and postoperative morbidity must be accepted [15, 21, 24, 25].
If a borderline resectable arterial encasement has been diagnosed preoperatively and true arterial infiltration is present during surgical exploration, a case-specific decision has to be made since no clear recommendations are available. In principle, a palliative treatment should be considered prior to any other approach. In some cases, neoadjuvant therapy and consecutive re-exploration or even immediate vascular resection in clinical trial settings can be evaluated [15].
If arterial involvement has been diagnosed preoperatively, neoadjuvant radio-/chemotherapy should be considered. Although rates of downstaging after neoadjuvant therapy have been reported to be low in most of the patients, R0 resections were possible due to an improvement of resectability [26–30]. However, the major problem after neoadjuvant therapy is proper restaging, since differentiation between vital tumor and fibrotic tissue continues to be difficult even with advanced imaging techniques like PET-CT [29, 31]. Hence, surgical exploration and immediate histopathological examination remains the only valid option in patients with regression or stable disease to get certain evidence of the nature of the remaining tissue and in consequence for the necessity and the extent of further resection.
If preoperative restaging demonstrates a progression of the disease, palliative treatment should be initiated.
15.4 Arterial Resections
Although already proposed by Appleby in 1953, resection of the celiac trunk or the superior mesenteric artery is still a rare condition in pancreaticoduodenectomies and should only be performed in high-volume centers by experienced surgeons [15, 32]. Emphasizing the latter statement, it could be demonstrated that patients being operated on in high-volume centers had more tumor-free resection margins [33]. Since perineural invasion is common in PDAC, the level of dissection should be at the adventitia; otherwise, the periarterial nerve plexus is not resected, which then leads to a significant decrease in survival [34, 35]. The approach of extended vascular resection is also supported by the study of Rehders et al. who confirmed that there is no correlation between vascular spread and the incidence of the spread of tumor cells. So arterial involvement does not predict a more aggressive tumor biology, but reflects the poor localization of the disease [36].
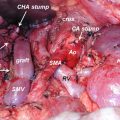
15.4.1 Superior Mesenteric Artery (SMA)
The area most infiltrated by the tumor is the margin toward the SMA [37]. Because of this, the “artery-first” procedure was developed. In general, before mobilizing the pancreatic head, the SMA is identified and dissected alongside its anatomical location. Then the neck of the pancreas and the stomach is separated. For this approach, several techniques have been proposed so far. Some start with a left-sided and others with a right-sided arterial resection and some with a supracolic approach [38–42]. Difficulties with the technique can occur in obese patients and with large tumors. In most cases, the SMA is reconstructed with a saphenous vein graft; however, transposition of the splenic artery is also an option. In addition, the “artery-first” approach has not only been proposed in conventional pancreaticoduodenectomies but also in field of minimal invasive surgery [43].
The “artery-first” technique allows identifying true involvement of the SMA before committing an irreversible step during surgery. It also provides a good balance between achieving tumor-free resection margins and preserving the nerve plexus around the SMA, which is important to avoid postoperative diarrhea. Except for the latter reasons, the approach also provides an early vascular control of the SMA and the SMV.
One of the common hepatic artery variations is the replacement of the right hepatic artery, which directly arises from the SMA (Michels III) and is located behind the pancreatic head. In addition, the common HA can also be completely replaced by a vessel, which directly originates from the SMA (Michels IX) [44–46]. These conditions should be known preoperatively. The “artery-first” approach allows early identification and handling of vascular anomalies [47].
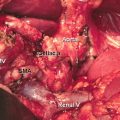
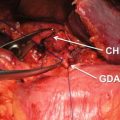
15.4.2 Celiac Trunk and Common Hepatic Artery
Distal splenopancreatectomy with resection of the celiac trunk (CT) or the common hepatic artery (HA) is performed more frequently in borderline resectable PDACs. Several modifications of the original Appleby’s procedure have been published [48, 49]. The CT or HA is resected in approximately 20% of all arterial resections performed for advanced PDACs [50]. The challenge of resecting the CT or HA is to simultaneously sustain perfusion of the liver, which should be taken over by the collateral flow through the pancreaticoduodenal arcade and the gastroduodenal artery. When performing Appleby’s procedure, a constant monitoring of the vascularization of the liver is mandatory. This can be achieved by assessment of the color and tension of the liver supported by intraoperative Doppler ultrasound. The arterial flow should be greater than 22 cm/s in order to prevent liver ischemia, postoperative liver failure, and infection, which could lead to biliary complications [51, 52]. The celiac trunk can be resected down to its origin from the aorta. In case of insufficient perfusion by the collaterals or necessity of total pancreaticoduodenectomy due to oncologic reasons, reconstruction is possible as long as the proper HA can be preserved. Reconstruction can either be done by primary tension-free end-to-end anastomosis or by using a venous, arterial, or synthetic graft [48]. The right gastric and gastroepiploic arteries should be conserved to secure adequate perfusion of the stomach.
15.4.3 Outcome
Few studies with a small numbers of patients included have evaluated the outcome after arterial resection [13, 37, 50, 53]. In general, the analyzed data is heterogeneous, and most of the conducted studies were non-randomized and retrospective. So reliable data on this topic are still missing. The meta-analysis of Mollberg et al. demonstrated that arterial resection is associated with increased perioperative morbidity and mortality as well as with a reduced 1-year survival as compared to the patients with venous vascular resection. Though, patients with advanced PDAC and arterial resection showed a better survival as compared to patients with advanced PDAC without any surgical intervention [13, 53]. Another study supports the results of the latter meta-analysis in regard to more tumor-free margins and better patient survival after arterial resection [37]. In summary, arterial resection should only be performed in high-volume centers by experienced surgeons and only in selected patients. Thereby, similar survival rates between vascular resected and nonvascular resected patients of up to a median of 18 months can be achieved [54, 52].
15.5 Venous Resections
15.5.1 Superior Mesenteric Vein and Portal Vein
Resection of the superior mesenteric and portal vein has gained concurrent acceptance to achieve tumor-free margins in centers for pancreatic surgery over the last decades. If suspicion of venous involvement has been raised in preoperative imaging, the subsequent vessel should be treated as truly involved during surgery to avoid experimental dissection and potential opening of the tumor. The localization of the tumor plays an important role in further management. If tumor involvement is close to the confluence of the splenic and mesenteric vein, resection and consecutive reconstruction can be performed more easily since the lumen is large enough to create a reliable anastomosis. The farther distal the tumor is located from the confluence the more limited the technical options in resection and reconstruction of the SMV are [15].
Depending on the relation of the tumor to the vein, varying techniques in venous resections have been performed. If only minimal contact to the vessel is present, a partial venous excision with direct closure (venorraphy) or closure by a patch is suitable.
In general, if local resection of the SMV is not possible, the mesenteric root should be mobilized completely by dissecting the retroperitoneal adhesions of the right hemicolon [55]. This provides great maneuverability of the SMV and consecutively improves alignment for anastomosis.
Segmental resection with primary venovenous anastomosis or segmental resection with interposed venous graft is necessary if tangential resection of the vein is not possible [56, 57]. The clamp time of the SMV and PV should be kept to a minimum to reduce edema of the bowel. The vascular graft can either be autologous (e.g., saphenous vein) or synthetic (e.g., goretex). Implanting a synthetic graft saves operation time, since no additional venous harvesting is needed, but bears the risk of every synthetic material in regard to infection and anastomotic leakage. Especially in the setting of an increased risk for developing pancreatic fistulas, graft infection can become chronic and eventually lead to hemorrhage. Though, in a series of 110 patients, no differences in morbidity and mortality between different types of portal vein reconstruction techniques were observed [58]. Since more evidenced-based data of venous reconstructions are needed, the ISGPS proposed a classification of venous resections to standardize nomenclature for improved recommendations in the future (see Table 15.2) [15].
Table 15.2
Proposed ISGPS classification of venous resections
Type | Classification |
|---|---|
I | Partial venous excision with direct closure (venorraphy) by suture closure |
II | Partial venous excision using a patch |
III | Segmental resection with primary venovenous anastomosis |
IV | Segmental resection with interposed venous conduit and at least two anastomoses |
15.5.2 Outcome
In two systematic reviews, no differences in morbidity and mortality have been observed for patients undergoing pancreaticoduodenectomy with and without venous resection [25, 59]. The latter studies demonstrated 3-year survival rates of 16–19.4% and 5-year survival rates of 7–12.3%, which are better than palliative treatment alone. The most important factors for survival were depth and the length of venous involvement and tumor-free resection margins. Patients with less than 3 cm of tumor involvement yielded a better survival than patients with more than 3 cm of tumor attachment. In addition, true involvement of the intima seems to be a factor of poor prognosis even in R0 resections [60–62]. After pancreaticoduodenectomy with venous resection, patients should receive adjuvant chemotherapy to further improve survival rates [63].
Venous resection during surgery for pancreatic cancer has become a standard procedure in recent years and has been included in several guidelines for pancreatic surgery. It should be performed on a routine basis by experienced surgeons if complete resection of the tumor can be achieved.
Conclusion
Tumor infiltration either venous or arterial is no contraindication for resection in PDAC anymore. Extensive preoperative workup with regard to vascular anatomy and surgical tolerance is mandatory. Correct staging after neoadjuvant therapy remains challenging, but is essential for patient selection to further improve long-term outcomes. Especially arterial resections should only be performed in selected patients in high-volume centers. Further prospective randomized trials are needed to elucidate the benefits of arterial resections.
References
1.
Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, et al. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann Oncol. 2013;24(10):2657–71.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree