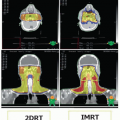
For any given individual patient with lymphoma, the precise etiology is usually unknown. However, risk factors for developing lymphoma have been identified. There is evidence for genetic susceptibility in lymphoma. Siblings of younger patients (<40) with HL have a 7-fold increased risk, and identical twins have a 100-fold risk.
4,5,6 Relatives of patients with NHL also appear to be at 4- to 10-fold increased risk of developing NHL.
5 Exposure to various substances including phenytoin, phenoxy herbicides (Agent Orange), hair dyes, dioxin, and benzenes has been found to have some correlation with an increased incidence of lymphoma.
7,8,9,10 Occupations in which exposure to these agents may occur are associated with a higher-than-normal risk for lymphoma; these include woodworking industries, agriculture, rubber and petrochemical industries, and dry-cleaning occupations. In contrast, exposure to ultraviolet sunlight (but not dietary vitamin D intake) and diets rich in fruits and vegetables have found to be associated with a reduction in the risk of developing lymphoma.
11,12,13 Exposure to prior radiation is also associated with increased risks of lymphoma, although the risk appears to be mainly in men.
14In addition to environmental factors, a variety of infectious agents have been associated with lymphomagenesis. In particular, chronic antigenic stimulation is thought to play an important role in the pathogenesis of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma).
15 Of particular relevance to the head and neck setting,
Chlamydophila psittaci DNA has been found in 47% to 80% of patients with ocular adnexal MALT
lymphoma,
15 and eradication with doxycycline has been shown to be effective in some patients.
16 Other infectious agents associated with MALT lymphoma include hepatitis C (splenic),
Helicobacter pylori (gastric), and
Borrelia burgdorferi (cutaneous). Patients with chronic hepatitis B also appear to carry an approximately threefold increased risk of development of NHL.
17 Other viruses associated with lymphoma include human T-cell leukemia/lymphoma virus (HTLV)-1, Epstein-Barr virus (EBV), human herpes virus (HHV) 8, and HIV.
18,19 Immunosuppression, whether acquired (HIV infection, 11-fold),
20 congenital (combined variable immunodeficiency, 12-fold),
21 or iatrogenic (organ transplant recipients, up to 240-fold),
22 is probably among the most potent risk factors for the development of lymphoma. Finally, other disorders characterized by dysregulation of the immune system have been associated with development of lymphoma. An increased incidence of NHL has been noted in patients with rheumatoid arthritis, celiac disease, Sjögren syndrome, dermatitis herpetiformis, inflammatory bowel disease, and acquired angioedema.
23,24 Lymphoma of the thyroid has been associated with either antecedent diagnosis or concomitant histologic finding of Hashimoto thyroiditis in more than 90% of cases, although <1% of patients with Hashimoto thyroiditis will develop lymphoma.
25