Fig. 13.1
Clinical target volume (CTV) considerations
The extent of the CTV is dependent upon several factors. Increasing confidence in the delineation and control of subclinical disease may allow more conformal target volumes. IFRT involved field radiotherapy, ISRT involved site radiotherapy, INRT involved node radiotherapy
The CTV is then expanded to create the PTV to account for setup uncertainty and target motion. A margin for penumbra in three-dimensional conformal radiotherapy is also necessary. Thoughtful patient positioning and immobilization can minimize the PTV margin, thereby sparing normal tissues. In addition, internal target motion, most commonly encountered in the thorax and upper abdomen, can be addressed in a variety of ways. ICRU Report 62 (ICRU 1999) defines the internal target volume (ITV) as the CTV plus a margin to account for changes in target position and shape. When applicable, the ITV should be defined using 4D CT simulation and/or fluoroscopy. In cases where clinically significant irradiation of normal tissues may occur due to tumor motion, strategies to minimize the ITV margin should be employed. A variety of approaches exist including deep inspiratory breath hold, gating, active-breathing control, and abdominal compression, among others.
Finally, volumetric delineation of organs at risk (OAR) should be included in RT treatment planning. The dose delivered to critical normal structures should then be considered in treatment planning. When appropriate, motion of OARs and setup uncertainties should be accounted for utilizing a planning organ at risk volume.
13.2.2 Simulation
Patient setup and immobilization can significantly influence the exposure of critical normal structures to radiation. The RT dose, organs at risk, and treatment verification plan should be considered prior to simulation. Strategies that optimize reproducibility, patient comfort, and geographic avoidance of normal structures are best. Patient instructions, such as full bladder or empty stomach, may further optimize normal tissue avoidance.
With rare exception, a CT simulation should be completed for treatment planning. Contiguous 2.5–5 mm slices should be obtained through the intended treatment volume. The use of IV contrast during simulation should be personalized and may not be necessary, particularly if a patient has achieved a complete response to chemotherapy. For abdominal and pelvic locations, oral contrast is often helpful to delineate the bowels. As noted above, target motion should be assessed and incorporated into treatment planning. For superficial targets, bolus may be required and should be placed at the time of simulation. In some centers, simulation MR and/or PET may be obtained in the treatment position and fused with the CT for treatment planning.
When applicable, all diagnostic images that will influence target volume delineation should be electronically fused with the simulation CT. A key first step in treatment planning is manual verification of all electronic fusions. When fusion is not possible, for instance, due to positioning differences between scans, careful manual transfer with concurrent review of both datasets should be carried out by the treating radiation oncologist.
13.2.3 Treatment Technique
Standard techniques, 3D conformal RT (3DCRT) or electron therapy, should be used whenever possible. In select cases with critical normal tissues in close proximity to irradiation targets, advanced RT techniques may be advantageous. These include IMRT, volumetric arc-based therapy, and proton therapy. Recommendations regarding the best technique for an individual case cannot be made and high-level data are lacking.
The use of more conformal techniques, such as IMRT or arc-based therapy, often results in greater sparing of high dose to critical normal structures at the cost of greater low-dose irradiation. In addition, the potential for geographic miss may be greater. Vigorous treatment verification, with image guidance, for example, should be considered when advanced techniques are used.
In cases with superficial disease involvement (e.g., conjunctival or cutaneous disease), electron irradiation may be preferred. Machine-specific depth dose characteristics of commissioned electron energies should be used for clinical treatment planning. CT simulation is recommended when faithful dose calculation algorithms (e.g., Monte Carlo) are available. Electron therapy is discussed in more detail below.
13.3 Disease-Specific Recommendations
Building on the target delineation and treatment planning principles noted above, this section presents clinical scenarios and provides more detail regarding the nuances of RT design and delivery. For comparison, Table 13.1 lists the standard anatomic definitions of the involved field.
Table 13.1
Involved fields using osseous anatomy
Superior | Inferior | Medial | Lateral | Notes | |
|---|---|---|---|---|---|
Cervical | 1–2 cm above mastoid tip and chin | 2 cm below clavicle | Ipsilateral transverse process | Include medial 2/3 clavicle | Add laryngeal block after 19.8 Gy |
Mediastinum | C5–6 interspace | Lower of 5 cm below carina and 2 cm below pre-chemo disease | N/A | Post-chemo volume with 1.5 cm margin | Include hilum with 1 cm margin (1.5 cm if involved) |
Axilla | C5–6 interspace | Lower of tip of scapula or 2 cm below lowest axillary node | Ipsilateral transverse process | Flash axilla | Include lung block |
Para-aortic | Higher of T11 or 2 cm above pre-chemo disease | Lower of L4 and 2 cm below pre-chemo disease | N/A | Transverse process or 2 cm margin on post-chemo volume | Kidney blocks may be necessary |
Inguinal | Middle of the sacroiliac joint | 5 cm below lesser trochanter | Medial border of obturator foramen with 2 cm margin on pre-chemo disease | Greater trochanter or 2 cm lateral to pre-chemo disease |
Many of the principles discussed thus far are applicable to nearly all types of Hodgkin and non-Hodgkin lymphoma. In addition to disease-specific discussion, site-specific recommendations are also presented below. Both the treatment site and the disease entity should be considered in the delineation of the target volume. For a given site, however, many aspects of simulation and treatment delivery remain unchanged regardless of disease. While the following sections are not divided by site, the following list may be helpful to direct interested readers to site-specific discussion:
13.3.1 Classical Hodgkin Lymphoma
13.3.1.1 Overview
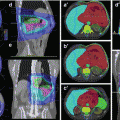
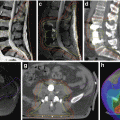
Until the 1990s, most patients with early-stage Hodgkin lymphoma were treated with full-dose (35–40 Gy) subtotal nodal irradiation, treating both involved nodal sites and adjacent uninvolved areas. The addition of chemotherapy to radiotherapy was shown to decrease the risk of relapse (Ferme et al. 2007; Specht et al. 1998). Further randomized studies demonstrated that in the setting of systemic therapy, smaller radiation doses and fields were adequate (Bonadonna et al. 2004; Engert et al. 2003, 2010). Currently, the most effective treatment is chemotherapy, typically ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) followed by low-dose, conformal radiotherapy to originally involved areas (Aviles and Delgado 1998; Laskar et al. 2004; Meyer et al. 2012; Noordijk et al. 2005; Pavlovsky et al. 1988; Picardi et al. 2007; Wolden et al. 2012; Herbst et al. 2010). Most randomized studies that have evaluated RT in the context of a combined modality treatment program have utilized the involved field concept (Fig. 13.2).
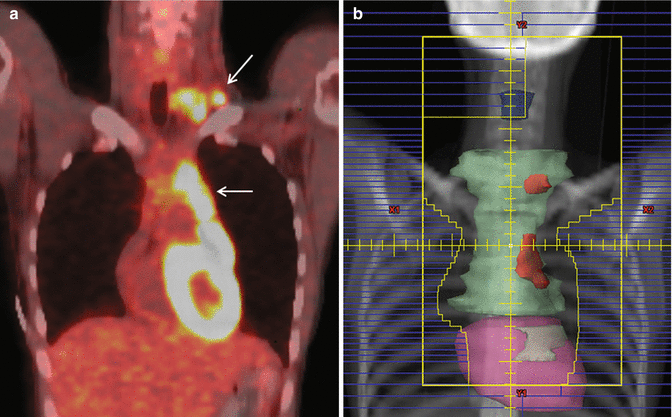

Fig. 13.2
Hodgkin lymphoma – involved field
Case | 22-year-old female with stage IIA nodular sclerosis Hodgkin lymphoma with erythrocyte sedimentation rate (ESR) of 65 (early-stage unfavorable) with complete response to four cycles of ABVD. Panel a – pre-chemotherapy PET/CT scan. White arrows indicate sites of disease involvement. Sites of involvement include the left neck, mediastinum, and pericardial lymph nodes |
Prescription | 23.4 Gy (1.8 Gy/fraction) |
Simulation | CT simulation without contrast, supine, free breathing, immobilized on indexed wing board with arms up |
Treatment planning | AP/PA treatment fields. Panel b – consolidation radiotherapy portal with custom multi-leaf collimators (MLC). Red volume represents residual CT abnormalities. Green volume represents the CTV. Blue volume is the larynx. Pink volume is the heart |
13.3.1.2 Consolidation RT
The principles of volumetric treatment planning discussed above can be used to define consolidation RT target volumes in early stage Hodgkin lymphoma. The volume of the CTV should be defined with careful consideration of the factors listed in Fig. 13.1. In general, the superior and inferior extent of the CTV should include the pre-chemotherapy extent of disease with 2 cm margin regardless of treatment response. The post-chemotherapy width, particularly in the mediastinum and retroperitoneum, is utilized to define the CTV. In cases where there is concern for residual disease, a shrinking field boost technique may be considered.
The treatment fields utilized in Hodgkin lymphoma are presently in flux. Three treatment options are shown in Fig. 13.3 for a patient with early stage, favorable Hodgkin lymphoma involving the left supraclavicular and axillary lymph nodes receiving consolidation RT after two cycles of ABVD. Treatment of an anatomically defined target volume, IFRT, is shown in panel a (see Table 13.1). ISRT and INRT are shown in panels b and c, respectively. Compared to IFRT, the ISRT field includes the axial extent of the involved lymph node stations with smaller superior-inferior margins and no prophylactic irradiation of uninvolved adjacent regions. In this case, the more generous IFRT was used as indicated by the recently published German HD10 study (Engert et al. 2010). In this study, only two cycles of ABVD were administered, and radiation therapy was given to an involved field. Whether involved site or involved-node radiotherapy is adequate in patients receiving only two cycles of ABVD is not clear.
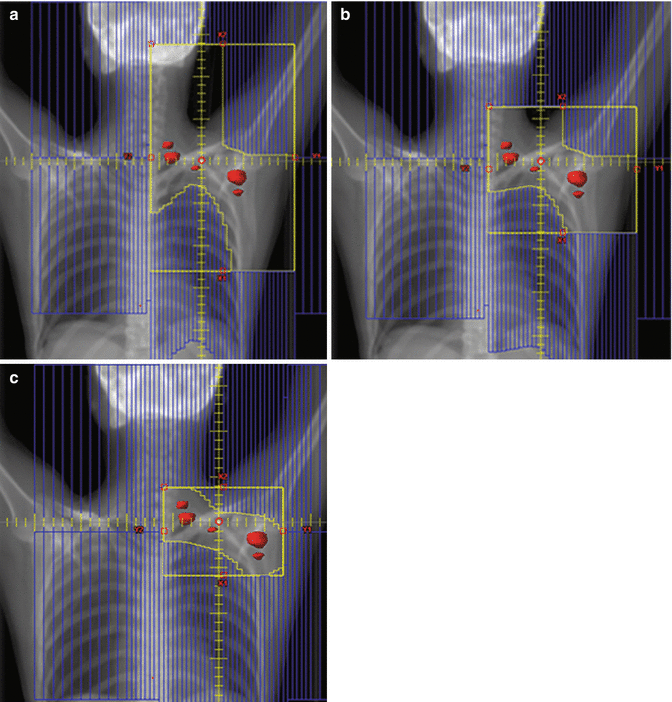

Fig. 13.3
Hodgkin lymphoma – IFRT, ISRT, INRT
Case | 26-year-old female with favorable stage IIA nodular sclerosis Hodgkin lymphoma with ESR 20 (early-stage favorable) with complete response to two cycles of ABVD |
Prescription | 19.8 Gy (1.8 Gy /fraction) |
Simulation | CT simulation with contrast, supine, free breathing, immobilized on indexed wing board with arms up |
Treatment planning | AP/PA treatment fields. Pre-chemotherapy GTV shown in red contour. IFRT includes the ipsilateral cervical, supraclavicular, and axillary anatomic regions (panel a). The superior and inferior extent of irradiation is reduced in ISRT (panel b). INRT includes only initial sites of disease (panel c). IFRT used in this case due to two cycles of chemotherapy |
13.3.1.3 Subtotal Nodal Irradiation (STNI)
Though infrequent, full-dose STNI is still employed in the contemporary management of Hodgkin lymphoma. A recent trial showed no difference in progression-free or overall survival in patients with favorable disease treated with either ABVD or STNI (Meyer et al. 2012). However, a combined modality approach is still generally pursued given the known late effects of STNI. More commonly, STNI is pursued when a patient has localized (stage I-II) disease that is refractory to multiple chemotherapy regimens.
The initial treatment fields are anatomically defined (Table 13.1) and include the lymph nodes in the bilateral cervical, supraclavicular, axillary, mediastinal, and hilar regions (mantle field). A dose of ~30 Gy to this area is followed by a ~10 Gy boost to sites of gross disease. Prophylactic treatment of para-aortic lymph nodes and the spleen typically follows after a 2-week break. Figure 13.4 shows a standard STNI field setup. Strategies to optimize accurate matching of supra- and infradiaphragmatic fields are needed. In the presented case (Fig. 13.4), a complete response was achieved after STNI, and the patient was then considered for a stem cell transplant.
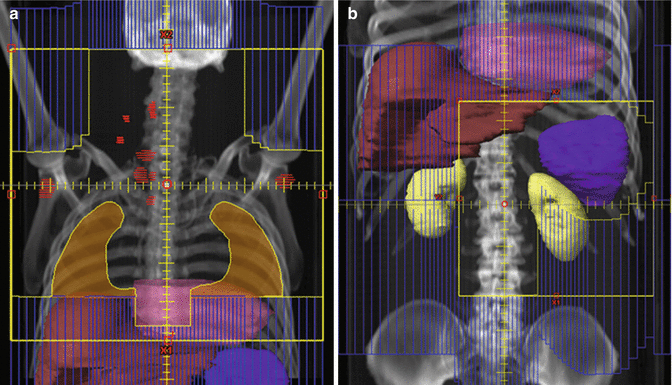

Fig. 13.4
Hodgkin lymphoma – subtotal nodal irradiation (STNI)
Case | 26-year-old female with stage IIA refractory Hodgkin lymphoma with disease in left neck and mediastinum unresponsive to ABVD and ICE, undergoing definitive radiation therapy |
Prescription | Mantle field (30.6 Gy) followed by boost to involved areas (10.8 Gy); para-aortic/spleen field (25.2 Gy) using 1.8 Gy fractions |
Simulation | CT simulation without contrast, supine, head extended, free breathing, immobilized in customized body mold |
Treatment planning | AP/PA treatment fields. Panel a – mantle field. Boosts fields not shown. Panel b – para-aortic/spleen field |
13.3.2 Nodular Lymphocyte-Predominant HL (NLPHL)
13.3.2.1 Overview
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is an unusual subtype of Hodgkin lymphoma, consisting of ~5 % of cases. It has a distinct immunophenotype (CD15 and CD30 negative, CD20 and CD45 positive), presentation, and natural history. While overall relapse rates are similar between classical Hodgkin lymphoma and NLPHL, patients with the latter tend to relapse later and survive relapses better (Nogova et al. 2008; Diehl et al. 1999). The optimal treatment is not clear, and given the rarity of this disease, randomized trials will be difficult to perform. For patients with localized disease, RT alone is generally recommended (Nogova et al. 2005; Chen et al. 2010). However, at least one study has suggested that the addition of chemotherapy may be associated with better outcomes (Savage et al. 2011).
13.3.2.2 RT Considerations
The same principles of volumetric treatment planning should be applied to NLPHL. In cases where systemic therapy is not used, a more generous CTV is recommended. On the other hand, following a good clinical response to systemic therapy, more conformal CTV volumes may be more appropriate.
For instance, Fig. 13.5 shows two clinical target volumes for a patient with NLPHL involving a single left inguinal lymph node. In the presented case, no systemic therapy was utilized, and the larger clinical target volume was treated (panel a) with a boost to the smaller clinical target volume (panel b), as RT was utilized to eradicate both clinical and subclinical disease. Theoretically, however, if systemic therapy had been given, it may have been more appropriate to irradiate the smaller CTV (panel b). For treatment of the inguinal region, the patient should be positioned frog-legged in order to separate the leg from the external genitalia and minimize skin folds. When possible the genitalia should be positioned out of the field, and the testicles should be shielded in men.
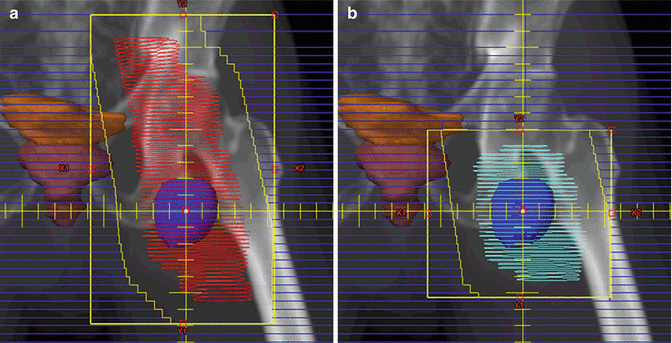

Fig. 13.5
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL)
Case | 52-year-old male with stage IA NLPHL involving a single left inguinal LN (dark blue contour). Treated with radiation therapy alone. Therefore, the initial clinical target volume includes the low pelvic and inguinal lymph nodes with a boost to gross disease |
Prescription | 30 Gy (2 Gy/ fraction) to CTV1 (red contour) (a); 2 Gy qd to 6 Gy to CTV2 (aqua contour) (b); total dose −36 Gy |
Simulation | CT Simulation without contrast, supine, with legs frog-legged. For male patients, position genitalia out of field and shield testicles with clam shell |
Treatment planning | 2-field 3D (AP and PA). The bladders (brown contour and orange contour) are adequately spared |
13.3.3 Diffuse Large B-Cell Lymphoma
13.3.3.1 Overview
Historically, RT was the preferred treatment for localized diffuse large B-cell lymphoma (DLBCL). Single-institution studies demonstrated that long-term disease control was achieved in ~40–45 % of patients (Vaughan Hudson et al. 1994; van der Maazen et al. 1998; Kaminski et al. 1986). Subsequent randomized trials demonstrated that the addition of chemotherapy to RT significantly decreased the risk of disease recurrence (Landberg et al. 1979; Monfardini et al. 1980; Nissen et al. 1983). Currently, the majority of patients are treated initially with immunochemotherapy, mostly commonly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Most (Horning et al. 2004; Martinelli et al. 2009; Miller et al. 2001), but not all (Bonnet et al. 2007), studies have shown that consolidation RT decreases the risk of recurrence after chemotherapy, and a combined modality approach has become a standard of care in localized DLBCL.
13.3.3.2 RT Considerations
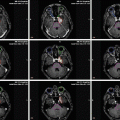
The principles of target volume delineation and treatment are similar to that of Hodgkin lymphoma for nodal DLBCL. DLBCL involves extranodal sites more commonly than Hodgkin lymphoma. When treating extranodal sites, clinical judgment is necessary to determine how much of the structure should be included in the clinical target volume. This will depend on the site treated as well as quality of diagnostic imaging. Figure 13.6 depicts the treatment plan for a patient with stage IIA DLBCL involving the left tonsil and ipsilateral level 2 neck. An ISRT CTV was delineated using the pre-chemotherapy PET/CT to include the entire tonsil and ipsilateral neck. Due to the proximity of the CTV to critical normal structures, namely, the parotid gland and oral cavity, IMRT was utilized to improve conformality. The prescription isodose contour is shown in cyan with simultaneous coverage of the CTV (red contour) and avoidance of the left parotid (yellow contour) and oral cavity (pink contour). IMRT is often advantageous for irradiation of targets in the head and neck due to proximity of many dose-limiting normal structures.
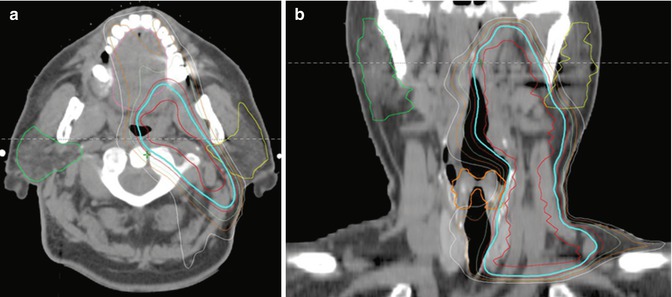
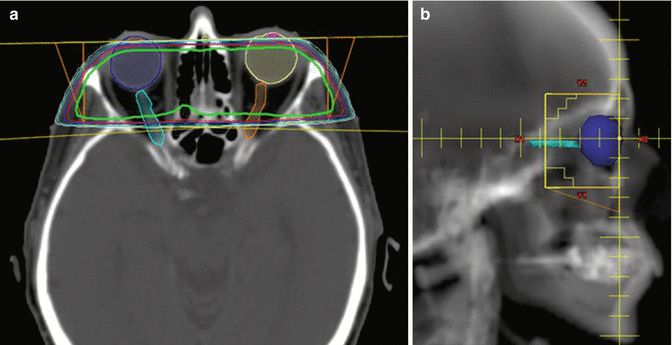
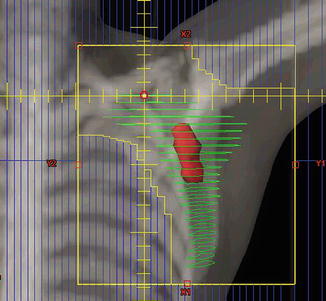

Fig. 13.6
Diffuse large B-cell lymphoma (DLBCL)

Fig. 13.7
Primary intraocular lymphoma

Fig. 13.8
Follicular lymphoma (FL)
Case | 72-year-old female with stage IA FL involving the left axilla staged by PET/CT and bone marrow biopsy |
Prescription | 24 Gy (2 Gy/fraction) |
Simulation | CT simulation without contrast, supine, immobilized on indexed wing board with arms up |
Treatment planning | 2-field 3D (AP and PA). The GTV is delineated in red and the CTV in green. Custom MLC blocking is utilized to irradiate the involved lymph nodes and regions at risk of harboring microscopic disease in the involved region |
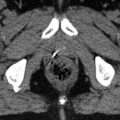
Figure 13.7 shows the opposed lateral treatment fields utilized in the consolidative irradiation of a patient with ocular DLBCL. In this case, the retina and vitreous were the targets for irradiation. The isocenter of treatment was placed at the anterior edge of the globe in order to minimize tangential irradiation of the cornea. Tissue-compensating wedges were used to improve dose homogeneity.
Case | 62-year-old male with stage IIA DLBCL involving the left tonsil and a level II cervical lymph node s/p left tonsillectomy. He received six cycles of R-CHOP chemotherapy with complete response by PET/CT |
Prescription | 30 Gy (2 Gy/ fraction) |
Simulation | CT simulation without contrast, supine, head extended, immobilized with thermoplastic mask, with arms down. IV contrast can be considered |
Treatment planning | 9-field IMRT plan. The CTV includes the left tonsillar fossa and ipsilateral neck (red contour). Critical normal structures include the left and right parotid glands (yellow and green contour, respectively), the larynx (orange contour, panel b), and the oral cavity (pink contour, panel a). The prescription isodose line is shown in cyan |
13.3.4 Follicular Lymphoma (FL)
13.3.4.1 Overview
Follicular lymphoma is the second most common subtype of non-Hodgkin lymphoma. Most present with advanced disease and are primarily managed with systemic immunochemotherapy. Low-dose RT can complement systemic therapy by providing very effective palliation of symptomatic areas (4 Gy in 1–2 fractions). For the minority of patients with localized disease, most published guidelines recommend RT alone (24–30 Gy). This recommendation is based on multiple studies with long follow-up showing 10-year failure-free survival of ~50 % (Vaughan Hudson et al. 1994; Campbell et al. 2010; Guadagnolo et al. 2006; Mac Manus and Hoppe 1996), with few relapses occurring thereafter. A study using data from the Surveillance, Epidemiology, and End Results (SEER) program demonstrating superior survival in patients who receive RT for stage I follicular lymphoma supports this approach (Pugh et al. 2010).
13.3.4.2 RT Considerations
Diagnostic imaging, preferably contrast-enhanced PET/CT, should be used to delineate the GTV. In most cases, localized follicular lymphoma will be treated with RT alone. Thus, RT is relied upon to eradicate clinically apparent and microscopic disease in the immediate region. Hence, IFRT is preferred over INRT. Most patients with stage I follicular lymphoma present with disease in the inguinal region, axilla, or neck. Treatment of these sites, compared to those more centrally located, is associated with a low risk of morbidity. Figure 13.8 depicts the GTV (red contour) and CTV (green contour) for a patient with stage IA FL of the left axilla treated with RT alone. The medial axillary lymph nodes are located where the axillary vessels cross the lateral aspect of the pectoralis minor. Since no systemic therapy was given, the medial lymph nodes are included as well as the lymph nodes in the supraclavicular fossa. Full humeral head blocking should be avoided when treating the axilla.
Case | 76-year-old female with bilateral intraocular lymphoma s/p high-dose methotrexate |
Prescription | 30 Gy (2 Gy/fraction) |
Simulation | CT simulation without contrast, supine, immobilized with thermoplastic mask |
Treatment planning | 2-field 3D plan (LAO, RPO). Opposed treatment fields were angled to match anterior divergence to minimize tangential irradiation of the cornea (panel a). Custom MLC blocking was utilized to target the posterior globe and distal optic nerves bilaterally (panel b). The prescription isodose line is shown in green. The right and left optic nerve are contoured in cyan and orange, respectively (panel a) |
13.3.5 Marginal Zone Lymphoma (MZL)
13.3.5.1 Overview
Marginal zone lymphoma is the third most common non-Hodgkin lymphoma and consists of three entities – extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), splenic marginal zone lymphoma, and nodal marginal zone lymphoma. The latter two entities are fairly rare and will not be discussed further. Most patients with MALT lymphoma present with localized disease. The most commonly involved sites include the stomach, orbital adnexa, salivary glands, thyroid, skin, and lungs (Goda et al. 2010). Though patients with localized MALT lymphoma are often treated with RT alone, the simulation and treatment planning approach vary widely. The most common sites of involvement receive additional attention below.
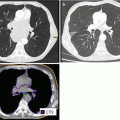
13.3.5.2 Stomach
The microorganism Helicobacter pylori is the causative agent of gastric MALT lymphoma for many patients. Eradication of the bacterium results in lymphoma regression and long-term disease-free survival in ~50 % of patients (Wundisch et al. 2005; Schechter et al. 1998). RT is used in those patients without an apparent H. pylori infection or who have refractory or progressive disease despite antibiotics. RT results in lymphoma eradication and long-term disease control in >90 % of patients.
RT Considerations
The target for irradiation is the entire stomach. Therefore, patients are asked to fast for 2–3 h prior to simulation and treatment to facilitate a smaller target volume. A small amount of oral contrast can be used to improve visualization of the stomach, and IV contrast should be considered in cases with concomitant regional lymph node involvement. Immobilization with a customized body mold is recommended. Respiratory motion can be assessed with 4D CT simulation and/or fluoroscopy. In general, more generous margins should be considered due to internal target motion in the treatment of gastric MALT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree