Assay
Gene/fusion transcript
Gene function
Test methodology/specimen
Test interpretation
Clinical utility
IGH-BCL2
Fusion of IGH-BCL2 t(14;18).
FISH
Presence or absence of fusion product. Presence or absence of BCL2 gene rearrangement
Presence of the translocation/fusion product supports the diagnosis of follicular lymphoma (70–95 % of cases). May be present in DLBCL or BCL-U [54].
The test detects IGH-BCL2 gene fusion and/or BCL2 gene rearrangement
qPCR (for monitoring therapy and MRD).
Other less common BCL2 translocation include: t(2;18)(p11;q21.3) and t(18;22)(q21.3;q11)
qPCR result may be used in MRD.
Break-apart analysis of the BCL2 gene rearrangement
Specimen: B, BM, FF, FFPE, or TI
IGH-MYC
Fusion of IGH located on 14q32 with MYC located on 8q24;t(8;14)(q24;q32)
Translocation of IGH (14q32) to MYC (8q24). MYC is a transcription factor, directs cellular functions related to growth, cell cycle progression, and survival.
FISH
Presence or absence of the translocation.
MYC rearrangement uses break-apart probe.
Specimen: FFPE or TI
Absence of translocation does not entirely exclude BL as there are other rare translocations: t(2;8)(p11;q24); MYC-IGK and t(8;22)(q24;q11); IGL-MYC [2].
For break-apart probe: presence or absence of 8q24 rearrangement is identified
IGH-BCL1
Fusion of IGH located on 14q32 with BCL1 (also known as CCND1) located on 11q13; t(11;14)(q13;q32).
CCND1 encodes protein that belongs to the cyclin family important in cell cycle regulations and regulation of CDK kinases. Mutations or overexpression of this gene alters the cell cycle and appears to be tumorigenic.
FISH
Presence or absence of translocation.
Useful in the diagnosis of mantle cell lymphoma (MCL).
PCR and qPCR
Specimen: FF, B, BM
FFPE or TI for FISH
BCL6
BCL6 rearrangement using BCL6 break-apart probe.
BCL6 regulated genes are involved in cellular proliferation and differentiation. BCL6 also inhibits DNA repair pathways and TP53. BCL6 prevents terminal B-cell differentiation through repression of PRDM1.
FISH
Positive or negative for BCL6 rearrangement.
Specimen: B, BM, FFPE, TI
ALK
ALK (2p23) and fusion partners.
ALK is a RTK belonging to the insulin receptor superfamily and is normally silent in lymphoid cells. ALK is normally expressed in the nervous system during embryogenesis but only focally in the adult brain and is suspected to be involved in neuron differentiation, mitogenesis. ALK receptor is inactive in the absence of engaging ligands and its expression results in increased apoptosis. ALK activation either ligand-mediated or as a result of ALK fusion proteins decreases apoptosis. Aberrant expression and/or function of ALK are known to promote tumorigenesis in several cancers. Nucleophosmin (NPM1) gene is an abundant nucleolar phospho-protein that shuttles between nucleolus and cytoplasm; it is involved in numerous cellular processes including genomic stability [2, 120].
RT-PCR for the t(2;5)
Positive or negative. Positive findings result in diagnosis of ALK positive ALCL. Negative findings result in diagnosis of ALK negative ALCL.
Commonly seen in anaplastic large cell lymphoma. ALK positive ALCL demonstrates better prognosis compared to ALK negative cases [2].
Most common fusion partner is NPM1 (5q35) fusion.
FISH: ALK break-apart probe
Standard RT-PCR may not detect variant translocations.
ALK fusion partners may be seen in non ALCL tumors like lung cancers. The fusion partners are different from those in ALCL.
Other fusion partners include TPM3 (1q25), ATIC inv(2)(p23q35).
Specimen: BM; FFPE.
IGHV mutation
Mutations of Immunoglobulin heavy chain variable (IGHV) located on 14q32.33 for CLL/SLL
Somatic hypermutations in the rearranged variable regions of the immunoglobulin heavy chain (IGHV) associated with subgroup of CLL/SLL and Mantle cell lymphoma patients.
RT-PCR/sequencing
Positive or negative.
Used for prognostic purposes. Patients with IGHV mutation appear to have a longer survival compared to patients without IGHV mutations. Unmutated IgVH appears to be an independent adverse prognostic marker in patients with CLL.
Specimen: B, BM
DNA sequences of B cells differing by 2 % or more from its germline counterpart are considered mutated; while those different by less than 2 % are considered unmutated.
Not used for diagnosis. Assay is designed for use most often in individuals with a confirmed diagnosis of CLL/SLL.
B-cell clonality
B-cell clonality
B lymphocytes are part of the immune system whose function is to protect the body against pathogens, thus generating enormous repertoires of antigen receptors (AgR). AgR diversity is restricted to the variable regions of the Ig and TCR proteins. This diversity is generated by variable (V), diversity (D) and joining (J) recombination or rearrangement during B lymphocyte development for the Ig heavy chain (IGH) and Ig light chain kappa/lambda (IGK/IGL).
PCR (gold standard): FFPE may be used; fresh/frozen tissue, relatively good sensitivity.
Positive or negative
Associated in most cases with B cell lymphoma.
Southern blot: requires high quantity of DNA and is labor intensive. Not commonly performed.
Negative result does not exclude lymphoma as neoplastic tumor cells less than the stated sensitivity may not be detected. Additionally somatic hypermutation can lead to false negative result for IGH, and thus IGkappa may be used.
Results must always be correlated with clinical and histomorphologic/cytomorphologic features of the specimens.
Positive results do not always indicate malignant lymphoma: Clonality is not equivalent to malignancy. Some clinically benign proliferations may have clonal proliferations, commonly seen in immunodeficient patients, and autoimmune patients. Specimens with few B cells present may show false positive results.
T-cell clonality
T-cell clonality
T lymphocytes are part of the immune system whose function is to protect the body against pathogens, thus generating enormous repertoires of antigen receptors (AgR). AgR diversity is restricted to the variable regions of the Ig and TCR proteins. This diversity is generated by variable (V), diversity (D) and joining (J) recombination or rearrangement during T lymphocyte development of the T cell receptor (TCR) gene loci, some of which contain different variable (V), diversity (D) and joining (J) segments which are subject to rearrangement.
PCR (gold standard): FFPE may be used; fresh/frozen tissue, relatively good sensitivity.
Positive or negative
Associated in a majority of the cases with a T cell lymphoma.
The rearrangement generally starts with a D to J rearrangement followed by a V to D-J rearrangement for TCR beta (TRB also known as TCRB) and TCR delta (TRD also known as TCRD). It involves direct V to J rearrangements for TCR alpha (TRA also known as TCRA) and TCR gamma (TRG also known as TCRG) [3, 121].
Southern blot: requires high quantity of DNA and is labor intensive. Not commonly performed.
Negative result does not exclude lymphoma as neoplastic tumor cells less than the stated sensitivity may not be detected.
Results must always be correlated with clinical and histomorphologic/cytomorphologic features of the specimens.
Positive results do not always indicate malignant lymphoma: Clonality is not equivalent to malignancy. Some clinically benign proliferations may have clonal origins, commonly seen in immunodeficient patients, and autoimmune patients (e.g., CD8+ T-lymphocytosis, initial phases of EBV+ lymphoproliferations in immunodeficient patients) [121].
BIRC3-MALT1
Fusion of apoptosis inhibitor 2 (BIRC3 also known as API2) on 11q21 and MALT lymphoma translocation gene 1 (MALT1) on 18q21, resulting in BIRC3-MALT1 t(11;18)(q21;q21)
BIRC3 (API2) belongs to the inhibitor of apoptosis family (IAP), which includes X-IAP, API1 (9c-IAP1) and ML-IAP.
RT-PCR
Presence or absence of the fusion product/translocation.
Associated with extranodal marginal zone lymphoma (MALT lymphoma). BIRC3-MALT1; t(11;18)(q21;q21) higher frequency of detection in pulmonary and gastric tumors.
FISH
BIRC3 (API2) inhibits the biological activity of caspases.
Specimen: frozen tissue; formalin-fixed paraffin-embedded tissue
Negative result does not exclude MALT lymphoma as most cases are negative. Other chromosomal translocations associated with MALT lymphomas include t(1;14)(p22;q32), t(14;18)(q32;q21) and t(3;14)(p14.1;q32).
Protracted remissions may be induced in H. pylori associated gastric MALT lymphoma by antibiotic therapy for H. pylori. However, cases with t(11;18) appear to be resistant to H. pylori eradication therapy.
MALT1 gene contains two immunoglobulin-like domains and a caspase-like domain (CLD), and is involved on antigen receptor mediated nuclear factor kappa B (NFκB) activation.
Associations of the other chromosomal translocations in MALT lymphomas include:
Wild type BIRC3 (API2) or MALT1 is incapable of activating NFκB, the BIRC3-MALT1 fusion product is capable of activating this transcription factor.
The t(14;18) translocation is detected in lung, ocular adnexae/orbit and salivary gland lesions.
T Cell Clonality
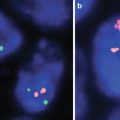
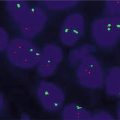
T cell clonality evaluation is similar to B cell processes with evaluation performed on the TCR gene. The TCR is a membrane bound heterodimer composed of two polypeptide chains, either αβ or γδ. PCR evaluation typically includes evaluation of the TCR gamma gene which is rearranged in a majority of αβ and γδ T cell lymphomas [4, 6]. An early report by Liu H. et al. showed that analysis of the TCR gamma gene had a clonality detection rate of 89–94 %. Additional evaluation of the TCR Beta gene yielded a detection rate of 94–98 % with the BIOMED-2 PCR assays [3, 5] (Fig. 18.1).
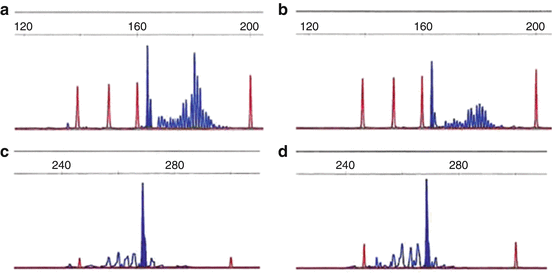

Fig. 18.1
T cell receptor PCR clonal findings. TCR PCR was performed on two separate skin lesions in an 18 year old male for evaluation of a cutaneous T cell lymphoma. (a) and (b) represent similar sized clones in TCR gamma for the right arm skin biopsy (a) and right lower extremity biopsy (b). (c, d) Similar sized clones were also seen in TCR beta for the right arm skin biopsy (c) and right lower extremity biopsy (d)
Role of Molecular Testing in High-Grade B Cell Lymphomas
High-grade B cell lymphomas are a group of B cell lymphomas characterized clinically as aggressive tumors. Although initially responsive to therapy in the adult population, a number of these patients relapse and eventually succumb to the disease [2]. Morphologically these tumors are composed of intermediate to large sized neoplastic lymphoid cells with round to irregular nuclei, vesicular to coarse chromatin, prominent nucleoli, and high ki-67 proliferation rate.
According to the 2008 World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Diffuse Large B cell Lymphoma (DLBCL) is the most common high-grade B cell lymphoma [2]. A majority of these cases are further subclassified as DLBCL not otherwise Text specified (DLBCL-NOS) which is composed of a number of cases that vary on their morphologic and immunophenotypic findings as well as clinical course.
Diffuse Large B Cell Lymphoma, NOS
In an effort to further subclassify DLBCL-NOS tumors based on molecular phenotype, Alizadeh et al. analyzed the gene expression profile of a number of B cell lymphomas and benign B cells [9]. The findings demonstrated that DLBCL cases could be further differentiated into two major prognostic groups: germinal center B cell like DLBCL (GCB-DLBCL) and activated B cell like DLBCL (ABC-DLBCL). GCB-DLBCL cases showed a similar expression pattern to benign germinal center B cells and ABC-DLBCL cases demonstrated a gene signature similar to activated peripheral blood B cells. A third group of cases corresponded to primary mediastinal (thymic) large B cell lymphoma and a lesser number of cases could not be further subclassified.
This cell of origin (COO) gene expression profile based classification demonstrated that the two distinct groups had significantly different clinical outcomes. Patients with GCB-DLBCL had an increased overall survival at 5 years compared to patients with ABC-DLBCL (76 % vs 16 %, respectively) after cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) therapy. Subsequent series have evaluated the clinical utility of this COO classification in patients treated with anthracycline based regimens, plus rituximab (R) and have shown similar overall survival differences [10–13].
Various studies have shown that the COO subtypes utilize different signaling pathways, differ in their B cell receptor (BCR) utilization, and contain gene specific mutations and chromosomal aberrations [14, 15]. These COO specific differences have been elucidated utilizing a number of molecular based platforms including gene expression assays, comparative genomic hybridization arrays, Sanger sequencing, fluorescent in situ hybridization (FISH), and next-generation whole-exome and whole-genome sequencing. Understanding the underlying molecular mechanisms of the COO subtypes has given rise to novel targeted therapeutics.
Diffuse Large B Cell Lymphoma, Germinal Center Type
GCB-DLBCL was initially found to have overexpression of genes associated with the germinal center such as BCL6 and CD10 [9]. Subsequent studies have demonstrated that mutations in epigenetic regulatory genes such as EZH2, KMT2D (MLL2), CREBBP, and EP300 may specifically affect GCB-DLBCL [16–19]. In one study, mutations in the EZH2 gene which encodes for a histone methyltransferase protein, was found in 20 % of GCB-DLBCL with none seen in ABC-DLBCL [20]. Mutations of histone acetyltransferase genes such as CREBBP and EP300 may also play a role in BCL6 activation and TP53 inhibition. In a study by Pasqualucci et al., 39 % of DLBCL contained genomic deletions and/or somatic mutations in both genes with genetic aberrations in CREBBP present in 41.5 % of GCB-DLBCL versus 17 % in ABC-DLBCL [21]. These findings indicate that targeting epigenetic modification pathways via histone deacetylase inhibitors may have future therapeutic efficacy. The combination of a proteasome inhibitor and pan histone deacetylase inhibitors have resulted in synergistic cell death in DLBCL cell lines [22].
The PTEN protein is a tumor suppressor protein which negatively regulates the PI3K/Akt/mTOR pathway. Loss of PTEN expression was present in over 50 % of GCB-DLBCL cases [23]. Decreased PTEN expression can be a result of PTEN locus deletions, somatic mutations, and posttranscriptional or posttranslational regulation via microRNAs [15].
Diffuse Large B Cell Lymphoma, Activated B Cell Type
Gene expression studies have shown that ABC-DLBCL has a gene signature similar to a post-germinal center B cell. A number of signaling pathways are implicated, many of which affect the NF-κB pathway. The NF-κB pathway has been shown to be constitutively activated in ABC-DLBCL and to a lesser extent in GCB-DLBCL [24, 25]. The NF-κB pathway is a transcriptional signaling pathway which is under the regulation of the B-cell receptor (BCR), tyrosine kinase pathways, and protein degradation pathways such as the ubiquitin–proteasome system [24–26]. NF-κB pathway activation results in the transcription of proteins involved in a number of cellular processes including tumor cell survival, proliferation, and metastasis [25].
The BCR pathway is a major signaling pathway which is upstream of the NF-κB pathway (Fig. 18.2). It has been shown that ABC-DLBCL is dependent on chronic active BCR signaling [14, 27]. Next-generation sequencing (NGS) and other methodologies have found mutations in various proteins within this pathway [18, 28, 29]. The Bruton agammaglobulinemia tyrosine kinase (BTK) pathway is downstream of the BCR and results in the activation of the NF-κB (NFKB1) pathway. Key proteins involved in the BTK pathway include the Bruton Tyrosine Kinase and the protein complex CARD11/MALT1/BCL-10. Mutations in CARD11 are seen in approximately 10 % of ABC-DLBCL via NGS [30].
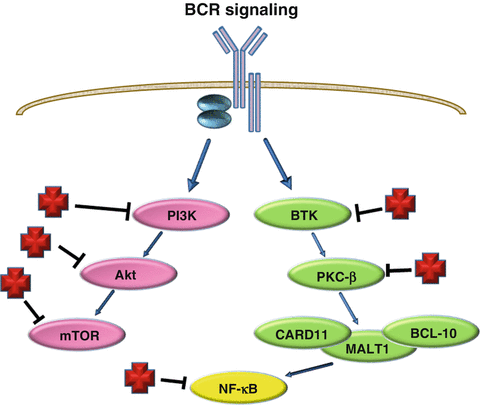

Fig. 18.2
Therapeutic targets in ABC-DLBCL related signaling pathways. ABC-DLBCL has chronic active BCR signaling with resultant activation of signaling pathways such as PI3K-Akt-mTOR pathway. Each protein of this pathway has specific targeted inhibitors. BCR also activates BTK which is inhibited by Ibrutinib which has been shown to have marked efficacy in a number of B cell lymphomas. Additional targets to NF-κB (NFKB1) are also routinely used in B cell lymphomas and plasma cell myelomas. ABC activated B cell, DLBCL diffuse large B cell lymphoma, BCR B-cell receptor. Red crosses represent targeted therapy currently under investigation
A number of genetic aberrations in DLBCL have also been elucidated through array comparative genomic hybridization (aCGH) and array single nucleotide polymorphisms (aSNP). Guo et al. found statistically significant differences between GCB-DLBCL which contained more gains at 7q22.1 and losses at 16q, and non-GCB DLBCL which contained more gains at 11q24.3 and 3q13.2 [31]. Lenz et al. analyzed 203 DLBCL cases and found upregulation of the SPIB and FOXP1 gene on chromosome 3 and deletion of CDKN2A (INK4a or ARF) in ABC-DLBCL [28]. Chen et al. analyzed a smaller sample of cases and found that loss of chromosome 1 was predictive of good outcome and gain of chromosome 12 was significantly associated with GCB-DLBCL [32].
COO Specific Targeted Therapy
Emerging drug regimens are targeting COO specific genetic aberrations due to the decreased efficacy of R-CHOP treatment for ABC-DLBCL. Targeted therapy with resultant inhibition of the NF-κB (NFKB1) pathway has shown marked efficacy in ABC-DLBCL [33, 34] (Fig. 18.2). Current therapy including Bortezomib indirectly affect the NF-κB (NFKB1) pathway through blockade of the ubiquitin–proteasome pathway. Upstream inhibition of the NF-κB (NFKB1) pathway via Ibrutinib targeting of the BTK (as well as other tyrosine kinases) and has shown marked efficacy in relapsed/refractory B cell lymphomas and ABC-DLBCL [15, 33, 35–37].
Double, Triple Hit B Cell Lymphomas
Another high-grade B cell lymphoma is B cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma (BCL-U) which includes tumors designated as “double” or “triple” hit lymphomas. These tumors contain translocations of the MYC gene with either a translocated BCL2 and/or BCL6 gene. It is important to differentiate these tumors from DLBCL and Burkitt lymphoma as the prognosis for double and triple hit lymphomas is poor and recent therapies demonstrate specific efficacies for each category [38].
All of the “Hit” tumors include a MYC gene translocation. The MYC oncogene is involved in proliferation and cell growth of tumor cells and is present in 3–16 % of DLBCL cases [39–47]. Double hit lymphomas are associated with a GCB-DLBCL phenotype and portend to a poor overall survival in a number of studies [48, 49] (Table 18.1).
BCL6, is a transcriptional regulator which is involved in proliferation, apoptosis, and DNA damage response [15, 50]. BCL6 expression is associated with GCB-DLBCL and increased protein and mRNA expression was associated with a better overall survival in DLBCL patients [51]. BCL6 translocations are more commonly seen in ABC-DLBCL.
BCL2 is an anti-apoptotic protein which plays a pivotal role in B cell lymphomas such as follicular lymphoma and DLBCL. The BCL2 protein is overexpressed when the BCL2 gene is translocated next to the IGH gene in the t(14;18)(q32;q21) which is seen in over a third of GCB-DLBCL cases [15, 52, 53]. Also amplification of 18q21 is associated with increased mRNA expression in ABC-DLBCL cases. In a study by Iqbal et al. there was a statistically significant association between increased BCL2 mRNA and protein levels in ABC-DLBCL and decreased overall survival [54]. NF-κB (NFKB1) pathway may also play a role in increased BCL2 expression levels [55].
Burkitt Lymphoma
Burkitt lymphoma is a high-grade B cell lymphoma seen in children and adults [2]. Children of Western countries typically have involvement of extranodal locations such as the ileocecal region. Morphologically, Burkitt lymphoma consists of intermediate sized lymphoid cells with round nuclei and prominent peripherally placed nucleoli. The lymphoma cells are characterized by expression of CD20 and CD10, contain a high ki-67 proliferation rate (approximating 100 %), and are mostly negative for BCL2 protein expression. Burkitt lymphoma is characterized by a MYC translocation typically involving the heavy chain gene on 14q32 but may also involve a translocation to the kappa or lambda light chain genes on chromosomes 2 or 22, respectively.
When to Test and Methodology
Molecular testing of high-grade B cell lymphomas play an essential role in determining the final diagnostic classification of the tumor, overall prognosis of the patient, and appropriate therapy. Analysis begins with the evaluation of the clinical, morphologic and immunophenotypic findings of the tumor. If the above findings indicate a Burkitt lymphoma, fluorescent in situ hybridization (FISH) confirmation of the MYC translocation should be performed. If there are findings which are not typical for Burkitt lymphoma (i.e., lower ki-67 proliferation rate, strong BCL2 protein immunohistochemical expression, pleomorphic morphology) FISH and karyotyping (if fresh tissue is available) are usually performed. FISH analysis for MYC, BCL2, and BCL6 translocations and when available, karyotyping to evaluate for a complex karyotype, is/are performed to rule out a BCL-U or DLBCL [2, 56] (Fig. 18.3) (Table 18.1).
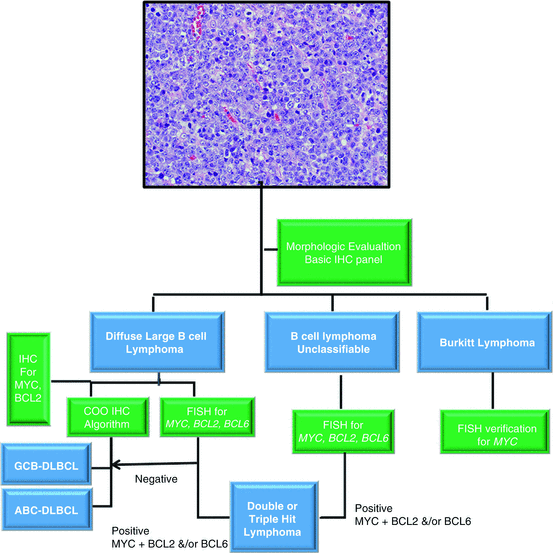

Fig. 18.3
Evaluation of a high-grade B cell lymphoma. Morphologic and immunophenotypic assessment are performed which confirms a mature B cell neoplasm and rules out a number of morphologic mimics. The tumor can be placed into one of three general categories: DLBCL, BCL-U, or Burkitt lymphoma. For the former two, FISH for MYC, BCL2, and BCL6 may be performed for the evaluation of a double or triple hit lymphoma. FISH for MYC may be performed for the confirmation of Burkitt lymphoma. For DLBCLs, evaluation of COO utilizing IHC algorithms should be performed. IHC for MYC and BCL2 overexpressors can also be performed if available
Cases of DLBCL should have the determination of COO performed if enough material is present. The original gene expression studies were performed on frozen tissue and not amendable to routine clinical practice due to the need for large quantities of RNA and lack of available frozen tissue. Newer methods utilize less quantity of RNA and can be taken from formalin-fixed paraffin-embedded (FFPE) material [57]. However, currently this is not routinely available in many laboratories. A number of immunohistochemical panel algorithms for the determination of the COO are used routinely and are further discussed in a later section.
Material permitting, DLBCL cases may also have routine FISH performed for the evaluation of MYC, BCL2, and BCL6 translocations to rule out a double or triple hit lymphoma, regardless of the immunohistochemistry determined COO. Although recent reports demonstrate that decreased CD20, CD19, and light chain expression may be indicators of a double hit lymphoma, currently there is no defined morphologic finding or immunophenotypic marker(s) which can accurately differentiate non-translocated DLBCL from a double or triple hit lymphoma [58, 59]. Thus it is currently debated as to which cases should be reflexed for FISH analysis. Some experts argue that increased protein expression of MYC, BCL2 and/or ki-67 should elucidate FISH analysis, while others feel morphology should determine FISH necessity. These guidelines will likely be addressed in the revised WHO classification.
Therapy
Determination of the COO for DLBCL allows clinicians to stratify these patients into certain prognostic and therapeutic categories. GCB-DLBCL have a better survival than ABC-DLBCL when treated with an R-CHOP regimen. Additional targeted treatments such as ABT-199 which targets the BCL2 protein, and inhibition of PI3K via PI3Kδ (PIK3CD) inhibitor Idelalisib have shown efficacy in a number of B cell lymphomas [60–63]. Patients with an ABC-DLBCL, as discussed previously, may currently benefit from a targeted approach to the NF-κB (NFKB1) pathway through the addition of Ibrutinib, bortezomib, and/or lenalidomide [33, 34, 64].
The aggressive double or triple hit lymphomas have a poor prognosis when compared to DLBCL and have shown increased progression free survival with intensive therapy regimens such as etoposide, vincristine, doxorubicin, cyclophosphamide, prednisone, and rituximab (DA-EPOCH-R) and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD) [15, 38, 49, 65, 66].
Immunohistochemical Testing
Immunohistochemical and/or flow cytometric analysis should be performed on all suspected high-grade B cell lymphomas. Since most laboratories are not performing GEP studies on these cases, COO is determined by immunohistochemistry panels. A number of algorithms for immunohistochemical evaluation have been generated such as the Hans, Choi and Tally algorithms [67–69]. Although IHC algorithms are not 100 % concordant with GEP studies, they will likely be used in many laboratories until a more cost-effective and time-efficient molecular platform is available [70].
A number of groups have evaluated the utility of BCL2 and MYC protein expression via immunohistochemical staining in determining DLBCL cases with decreased prognosis. One of the difficulties in utilizing the antibodies lies in the determination of the positive and negative cutoff values. Two of the recent studies determined a cutoff value for MYC at ≥40 %. The study by Johnson et al. determined the cutoff value via evaluation of the clinical findings of patients in the training cohort, while 40 % was chosen by Green et al. by determining it as the mean of all cases [49, 66]. The BCL2 cutoff level in both studies was 50 % and 70 %, respectively. In both studies patients with positive MYC and BCL2 staining were shown to have decreased overall survival in their training and independent validation patient cohorts in both ABC-and GCB-DLBCL. However, unlike FISH positive double hit lymphomas, cases which were positive for MYC and BCL2 expression (overexpressors) were more commonly of ABC type. A more recent study by Perry et al. calculated positive cutoff levels of 50 % for MYC and 30 % for BCL2. These levels were predictive of decreased OS and EFS in patients positive for both markers in their multivariate analysis. However, when they analyzed these markers with the specific COO groups, there was a statistical difference in OS and EFS in the GCB-DLBCL, but not in the non-GCB-DLBCL. They cited that this may be due to smaller sample size [71].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree