(1)
Institut Bergonié, Bordeaux, France
Abstract
The two types of lymphocytes, B and T, which are, respectively, responsible for humoral and cell-mediated immunity, express membrane receptors in relation to their immune functions: their task involves antigen recognition and elaboration of appropriate responses. Toward a multitude of possible antigens, a huge diversity in recognition structures is necessary: it is represented by the membrane immunoglobulins in charge of their binding. Contrasting to this diversity is the relative uniformity of receptor-associated proteins that are in charge of the elaboration of the response and are bound to the receptor itself by non-covalent bonds. Downstream the receptor complex, several pathways that have been studied in other chapters may be implemented to regulate lymphocyte proliferation and migration; they mainly involve the activation of cytoplasmic kinases, the generation of second messengers and the activation of small G-proteins.
We cannot summarise all immunology in a single chapter, but only extract some information related to the modalities of lymphocyte signalling, from receptors to effectors, trying to identify the features that cancer cells can utilise to reach their own goals and how these features can serve as potential targets for anticancer treatments.
13.1 B-Cell Receptors
13.1.1 B-Cell Receptor Activation
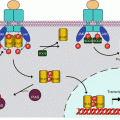
B-cell receptors (BCRs) are multiprotein complexes comprising a membrane immunoglobulin (Ig), which can bind the antigen and may be of infinite diversity, and a signalling element comprised of two constant proteins bound together via disulphide bridges, Igα (CD79A) and Igβ (CD79B). The antigen-binding element is a complete Ig, associating two heavy H chains and two light L chains, which is inserted in the plasma membrane through its Fc portion, while the N-terminal Fab portions are presented to the outside (Fig. 13.1). These Fab portions present a huge variety of sequences, obtained by gene recombination and somatic hypermutations; they determine the clonal specificity of antibodies, and each of them is able to recognise and bind an original antigen. The signalling element, Igα and Igβ, is associated to them through non-covalent bonds. Igα and Igβ both contain an activation motif containing two tyrosine residues (ITAM, immunoreceptor tyrosine–based activation motif) on the cytoplasmic side.
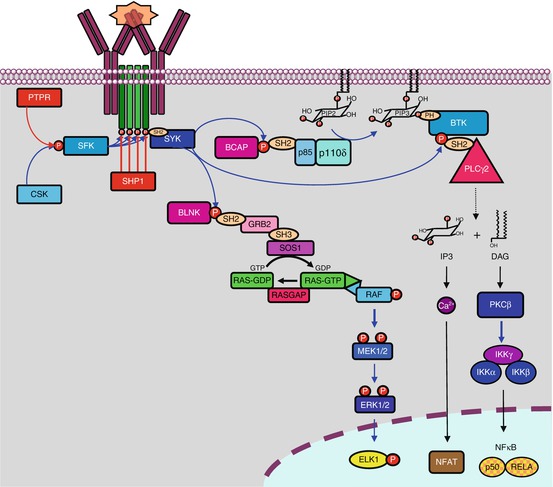

Fig. 13.1
B-cell receptors and associated signalling pathways. B-cell receptors are comprised of two immunoglobulin chains for ligand (soluble antigen) recognition and a complex formed of two α and two β chains for signal transduction. Phosphorylation of tyrosine residues of the α and β chains by LYN, an SRC family kinase, equilibrated by SHP phosphatases, allows the recruitment of another tyrosine kinase, SYK, which phosphorylates the adapter protein BLNK. BLNK induces the activation of proliferation pathways, thanks to the recognition of its phosphotyrosine residues by SH2 domain-containing proteins, of which an example is given (the MAP kinase pathway). Activated via BCAP (B–cell cytoplasmic adapter for PI3K), PI3Kδ plays a major role in activating the Bruton kinase, BTK, which in turn activates PLCγ2. PLCγ2 catalyses the formation of PIP3 and DAG from phosphatidylinositol 4,5-bisphosphate, and DAG activates PKCβ, a serine/threonine kinase at the origin of the production of active NFκB transcription factors through the phosphorylation of an adapter protein, CARD11, which forms a complex with two proteins, MALT1 and BCL10 (not shown). IP3 activates Ca2+ release from intracellular stores, which activates, among many other effects, the NFAT transcription factor
Cytoplasmic tyrosine kinases are in charge of the phosphorylation of these ITAM tyrosine residues (Fig. 13.1); this allows subsequent phosphotyrosine recognition by SH2 domain-containing proteins (see Chap. 1). These kinases belong to the SRC family kinases (SFK), especially LYN (Yamaguchi sarcoma viral related oncogene homologue), FYN (FES-YES-related novel gene), BLK (B-lymphoid tyrosine kinase) and LCK (lymphocyte-specific protein tyrosine kinase). These kinases are anchored to the plasma membrane thanks to a myristoyl chain added after translation. Their activity is facilitated by the local aggregation of the receptors in lipid rafts; this aggregation takes place upon receptor activation and enables kinase clustering. Phosphatases like SHP1 (SH2–containing phosphatase 1) can be recruited to counterbalance kinase activation and obtain an adequate level of phosphorylation of the ITAM tyrosine residues. Outside the reception complex, LYN is itself submitted to a balance between inactivating phosphorylation, mediated by CSK (C–terminal SRC tyrosine kinase), and activating dephosphorylation mediated by CD45 or PTPRC (protein tyrosine phosphatase, receptor type, C) (Chap. 1).
The ITAM phosphotyrosine residues of Igα and Igβ constitute docking sites for another tyrosine kinase, SYK (spleen tyrosine kinase), which afterward recognises and phosphorylates target substrates serving as effectors of BCR activation, among which is BLNK (B–cell linker protein) or SLP65, able to recruit various SH2 domain-containing proteins and to serve as a scaffold for diverse signalling molecules. The main early effector of BCR signalling pathway is a cytoplasmic kinase of the TEC family, called Bruton kinase (BTK). This kinase, which is equipped with SH2, SH3 and PH domains, is first attracted to the membrane by interaction of its PH domain with phosphatidylinositol 3,4,5-trisphosphate (PIP3) and then activated by phosphorylation on Tyr551 by SYK or SFKs. PIP3 is generated by PI3 kinase δ (Chap. 3), which is activated by the binding of the SH2 domains of its regulatory subunit, p55 (gene PIK3R3), to the phosphotyrosine residues of an adapter protein, BCAP (B–cell cytoplasmic adapter for PI3K, gene PIK3AP1), generated by SYK.
After recognition and binding, the BCR-activating antigen is internalised with the receptor and sent to endosomal compartments to be presented later to the major histocompatibility complex. Igα and Igβ are in contrast kept in the membrane so that they can sustain signalling in the absence of the reception complex. Figure 13.1 presents the initiation of BCR signalling.
13.1.2 BCR Signal Transduction Pathways
BCR activation leads to several signalling pathways (Fig. 13.1), especially aimed at cell proliferation required for the clonal expansion of B lymphocytes. We have already presented most of these pathways, and we refer the reader to the specific chapters where details are given:
The PI3 kinase pathway (Chap. 3), already mentioned, is activated through multiple ways: one of them involves the adapter protein BCAP and another one the adapter protein GAB2; these adapter proteins are phosphorylated by SYK, and the resulting phosphotyrosine residues are recognised by the SH2 domain of the regulatory subunits of PI3K. CD19, a membrane protein considered as a coreceptor for the BCRs, is also involved in PI3 kinase activation pathway via the adapter protein BCAP; in addition to BTK activation, mentioned above, the PI3 kinase pathway activates AKT and mTOR, in lymphocytes as in other cells.
The MAP kinase (ERK) pathway (Chap. 2) is activated through the recruitment of GRB2 by the adapter protein BLNK; GRB2 recruits in turn SOS1, the usual GEF for RAS proteins, which activates the MAP3 kinases of the RAF subfamily. Other GEFs activate the other MAP kinase pathways (p38, JNK) via the recruitment of the adequate MAP3 kinases.
The IKK pathway (Chap. 12), which leads to the production of NFκB transcription factors, is activated by several means: IKK can be activated either by AKT, downstream PI3 kinase, or by TAK1 through the activation of small G-proteins by recruitment of an SH3 domain-containing GEF; IKK activation can also occur in lymphocytes via a more complex way: BTK can activate a phospholipase C, PLCγ2 (gene PLCG2), recruited via binding the phosphotyrosine residues of BLNK; PLCγ2-mediated cleavage of phosphatidylinositol 4,5-bisphosphate generates diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (Chap. 6). IP3 activates calcium release from storage compartments to the cytosol, which activates in particular the NFAT transcription factor (Chap. 15), while DAG can in turn activate PKCβ, at the origin of the production of active NFκB transcription factors through the phosphorylation of an adapter protein, CARD11, which forms a complex with two proteins, MALT1 and BCL10, able to activate the IKK complex.
All these pathways converge toward transcription factors involved in lymphocyte survival and proliferation, which are studied in the corresponding chapters, such as MYC, ELK, JUN and above all NFκB, which appears as the main transcription regulator of BCR activation. BLNK phosphotyrosine residues can also be recognised by GEFs such as VAV1, which activate the small G-proteins of the RHO family (CDC42, RAC1 and others), which control cell motility by their actions on the actin cytoskeleton, via proteins of the WASP family (see below).
13.1.3 Oncogenic Alterations and Pharmacological Targets
Since the B-cell reception system is exclusively expressed in B lymphocytes, the only malignancies that could be associated to the deregulation of this pathway are lymphomas, which can occur either through specific alterations occurring in lymphocyte signalling pathways or through common alterations occurring specifically in lymphocytes.
It is suspected for more than 50 years that antigen stimulation can contribute to the genesis of malignant non-Hodgkin lymphoma, owing to chronic inflammatory states. In addition, antigenic stimulation of BCRs is required for B lymphocyte survival: as a consequence, the expression of a functional BCRs would be the way through which original molecular alterations (translocations) could be expressed in a transformed lymphocyte lineage. Lymphocyte proliferation would be dependent upon antigen stimulation, and indeed very few malignant lymphoma cells no longer express BCRs. This would explain why anti-infectious or anti-inflammatory treatments contribute to lymphoma regression; this is the case for lymphomas associated to the hepatitis C virus or to the bacteria Helicobacter pylori: in these situations, the antiviral or antibacterial treatment represents an efficient therapeutic option. More generally, any approach aiming at the elimination of the antigens involved in tumour cell expansion would be efficient.
In addition to this non-specific mechanism explaining the global involvement of BCRs in lymphoma genesis, molecular alterations of specific proteins involved in BCR signalling have been related to oncogenesis. Activating mutations of CD79A and CD79B have been identified in B-cell lymphomas. The tyrosine kinases of the SRC family as well as SYK do not seem to harbour common activating mutations in B-cell malignancies. BTK is crucial for the survival and proliferation of B cells; loss-of-function germinal mutations lead to X-linked agammaglobulinaemia (XLA), an immunodeficiency disease, but activating mutations have not been identified in lymphocyte proliferations. The CARD11 gene presents activating mutations that disconnect NFκB production from BCR activation, and the genes encoding its partners, MALT1 and BCL10, are rearranged in some B-cell lymphomas. As in common epithelial cancers, activating mutations in the MAP kinases and PI3 kinase pathways, when they occur in lymphocytes or lymphocyte progenitors, can contribute to oncogenesis.
Several potential therapeutic targets can be found in the BCR-activated pathway. SRC family kinases can be inhibited by various tyrosine kinase inhibitors (TKIs), such as dasatinib, bosutinib and saracatinib, but these TKIs are certainly not specific for the first kinase of the BCR pathway, LYN. A TKI developed for specific SYK targeting, fostamatinib, has entered clinical trials in non-Hodgkin lymphomas. The Bruton kinase benefits from a selective and irreversible TKI, ibrutinib, which has revealed a marked activity in malignant lymphoma, but not in those harbouring an activating mutation downstream BTK, such as those occurring in the CARD11 gene. Moreover, ibrutinib appears able to select resistant clones through mutations in BTK itself or in PLCG2. PKCβ is also a potential target in lymphomas; however, the clinical trials performed with enzastaurin or sotrastaurin have been disappointing. Of course, the non-lymphocyte-specific pathways activated downstream BCR activation can be used for lymphoma treatment; the corresponding inhibitors have been subjected to clinical trials but will not be detailed here: MEK inhibitors (Chap. 2), PI3 kinase inhibitors (Chap. 3), rapalogues (Chap. 3), NFκB downregulators (Chap. 12), etc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree