Author
Patients
Treatment
Oocytes recovered
LPS
FPS
LPS
FPS
von Wolff et al. [22]
12
28
rFSH-Antag
8.5
11.5
Bedoschi et al. [4]
2
rFSH-Antag
12
Maman (2010) [16]
5
13
IVM
150 FSH-10,000 IU hCG
12.8
17.3
Nayak and Wakim [19]
4
rFSH-Antag + GnRH bolus
14–4
Sönmezer et al. [21]
3
Letrozole 2,5 + rFSH
9–17
Cakmak (2014) [7]
22
93
35-R
Letrozole-CC-HMG-GnRH bolus
8.6
11
1.3.2 Luteal-Phase Stimulation in an Egg-Donation Programme
Although the viability of luteal-phase stimulation had been demonstrated, there were no data on the evolutionary potential of the embryos obtained. For this reason, Martínez et al. [17] performed a prospective study on egg-donation oocytes, with the main objective of evaluating the clinical pregnancy rate in recipients of vitrified oocytes obtained after donor stimulation from the initial luteal phase of the cycle, comparing it with that of oocyte recipients obtained after stimulation in follicular phase. In this study, nine egg donors were recruited who did two consecutive stimulation cycles, one conventional stimulation cycle and another in luteal phase, 3 months apart (Fig. 1.1).
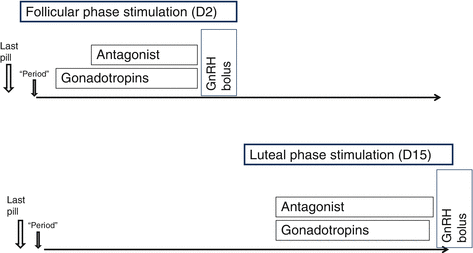

Fig. 1.1
Diagram of follicular phase stimulation protocol (day 2) and luteal phase (day 15)
After ultrasound evaluation and hormonal analysis, on day 2 of withdrawal bleeding after interruption of the contraceptive (D2), or on day 15 of post-withdrawal bleeding (D15), rFSH stimulation was started at a dose of 150–300 IU/day according to body mass index (BMI). Administration of the GnRH antagonist was added from the presence of a follicle >14 mm in the follicular phase, while in the luteal phase, it was started simultaneously with rFSH administration and was maintained until preovulatory triggering. Triggering took place with GnRH agonist when the presence of at least three 18-mm-diameter follicles was observed.
All the mature oocytes obtained after luteal-phase stimulation (D15) were vitrified. Following conventional stimulation (D2), some of the oocytes were vitrified and others were donated fresh. There were no differences in the dose of gonadotropins in the days of stimulation necessary or in the number of oocytes retrieved (Table 1.2).
Table 1.2
Dose of gonadotropins, days of stimulation, and number of oocytes retrieved among donors stimulated from day 2 or day 15
Donors | D2 | D15 |
|---|---|---|
Dose of rFSH (IU) | 2261 ± 940 | 2147 ± 535 |
Days of stimulation. | 10.44 ± 1.74 | 9.89 ± 1.2 |
No. of oocytes | 17 ± 6.65 | 22.5 ± 10.56 |
The unusual thing about this study is that it became possible to evaluate the two types of stimulation in the same population of women, since up to now, the two protocols had always been analysed in different groups of women.
The recipients received the standard treatment of endometrial preparation with estradiol valerate and vaginal progesterone. After warming the oocytes, they were inseminated with ICSI and one or two embryos were transferred on day 3 of embryo development. There were no statistically significant differences between the two groups in fertilisation, number of embryos transferred, and embryo quality. No differences were observed in the pregnancy rates per transfer (58.3 % in recipients of RD2 oocytes vs. 62.5 % in recipients of RD15 oocytes) (ns).
1.3.3 Luteal-Phase Stimulation in IVF Patients
Bearing in mind the outcomes obtained following random stimulation in patients for fertility preservation, an attempt was made to extrapolate the experience of LPS to IVF patients with the intention of developing a protocol that could be performed irrespective of the time of the cycle [6]. The authors performed a case–control study in which ten patients were treated with LPS (from days 19 to 21 of the cycle) and 30 patients with FPS (days 2–3 of the cycle) with rFSH and antagonist. They observed that a dose almost three times greater was required in the group treated with LPS. In both cases, the embryos were cryopreserved in 2PN with subsequent cryotransfer in artificial cycle, obtaining a pregnancy rate of 10 % in the LPS group compared with 61.3 % in the FPS group. The authors concluded that this concept was not applicable for routine use and its large-scale application should be studied further in fertility preservation patients.
Shortly afterwards, however, Kuang et al. (2014a) published the outcomes obtained in 242 IVF/ICSI patients after LPS, in which they froze all the embryos and later did the transfer in natural or artificial cycle. Stimulation was started immediately after confirmation of spontaneous ovulation and was done using an aromatase inhibitor (letrozole 2.5 mg/day) and hMG (225 IU/day). The authors point out that in this kind of protocol, there is no need for antagonist administration to inhibit the pituitary gland because there is no risk of endogenous increase in LH. They performed the final maturation administering a bolus of GnRHa (triptorelin 0.1 mg). No case of ovarian hyperstimulation was observed. Nor was there any premature increase in LH in the LPS cycles, compared to a 20 % increase observed among the cycles with conventional stimulation. A clinical pregnancy rate of 55.46 % (127/229) was obtained and a cumulative pregnancy rate of 64.7 % (112/173). At the time of publication, there were 48 births and 44 ongoing pregnancies, confirming the competence of the oocytes obtained after LPS and the viability of the embryos. This study is an important milestone because it was carried out in a large number of patients.
From the endocrine point of view, it is interesting that no premature increase in LH was observed among the LPS cycles, although there was no suppression of endogenous LH with the administration of a GnRH antagonist, compared with 27–25 % premature increase in LH among the follicular-phase stimulation (FPS) cycles, simplifying the need to monitor the treatment.
More recently, at the 2014 COGI Congress in Barcelona, Kuang (2014c) presented the current data from his group and announced that he and his group had given up on conventional stimulation and were routinely using LPS and transfer of cryopreserved embryos, with excellent outcomes.
1.3.4 Luteal-Phase Stimulation in the Low Responder
In 2013, Xu and Li [23] reported a case of “flexible ovarian stimulation” in a patient with low response in which, following intense ovarian stimulation and negative follicular aspiration, they continued the stimulation with FSH and clomiphene up to day 22. They retrieved one oocyte, which was fertilised and frozen and, after later cryotransfer, led to a pregnancy.
Later on, Kuang et al. [14] published their experience with the “Shanghai Protocol” of double stimulation during the follicular phase and the luteal phase in low response IVF/ICSI patients. The study was performed in 38 patients who met the Bologna criteria for low response. The first stimulation was performed with a combination of clomiphene citrate (25 mg/day) from the start until ovulatory triggering, letrozole 2.5 mg/day during the first 4 days, and hMG 150 IU every 2 days from that time until triggering with a GnRH agonist. They also added ibuprofen (600 mg/day) from the day of triggering to the day of aspiration to avoid early ovulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree