© Springer International Publishing AG 2018
Philip T. Cagle, Timothy Craig Allen, Mary Beth Beasley, Lucian R. Chirieac, Sanja Dacic, Alain C. Borczuk, Keith M. Kerr, Lynette M. Sholl, Bryce Portier and Eric H. Bernicker (eds.)Precision Molecular Pathology of Lung CancerMolecular Pathology Libraryhttps://doi.org/10.1007/978-3-319-62941-4_44. Lung Cancer Stem Cells
(1)
Department of Pathology, University of Texas Medical Branch, Galveston, TX, USA
Keywords
Stem cellsPluripotentialityMicroenvironmentNicheChemoresistanceSomatic, or adult, stem cells are another population of stem cells identified in human beings. Somatic stem cells have a limited capacity for self-renewal and play a role in tissue self-renewal [1–4]. Somatic stem cells are important for tissue repair and regeneration and have been identified in many tissues, including hematopoietic, neural, epidermal mammary, hepatic, mesenchymal, gastrointestinal, and pulmonary tissues. Adult stem cells include hematopoietic stem cells, typically found in the bone marrow, mesenchymal stem cells, and stem cells residing in specific organs, termed progenitor cells. Organ-specific progenitor cells are generally believed to aggregate in special tissue microenvironments, termed the stem cell niche [5–34]. These progenitor cell populations in the lung are thought to arise from differentiation of embryonic stem cells; however, these cells have also been considered to possibly arise from mesenchymal stem cells or hematopoietic stem cells [35–37].
With the study of cancer stem cells, the traditional “clonal evolution theory ” [38] has given way to the “cancer stem cell theory ” of carcinogenesis. The “clonal evolution theory ” proposes that each neoplastic cell within a tumor has an equivalent carcinogenic potential. It is under the auspices of this theory that modern chemotherapy and radiotherapy, attempting to destroy all cancer cells with a high proliferation capacity, are based. Unfortunately, under this theory, cancer cures, particularly with solid organ neoplasms, remains elusive for many cancer patients.
The “cancer stem cell theory ” proposes that uninhibited carcinogenic potential in malignant neoplasms is due to cancer stem cells, a rare subset of the overall tumor cell population with the ability to self-renew, differentiate into non-stem cancer cells, and produce new tumors via the formation of heterogeneous cell populations. Unlike tumor cells proposed under the “clonal evolution theory ,” these multipotent cancer stem cells are believed to produce intratumoral heterogeneity via aberrant capacity for differentiation [39–44]. Cancer stem cells are able to resist chemotherapy and radiotherapy, with resultant tumor relapse and poor patient prognosis. Prevention of tumor relapse and resultant potential cure depends, under the “cancer stem cell theory ,” on the destruction of the cancer stem cell population. The theory posits asymmetric division of cancer stem cells, as is also a characteristic of normal stem cells, which results in a daughter cell that retains the characteristics of stemness such as indefinite self-renewal and in a daughter cell committed to differentiation, having lost the characteristics of stemness [45–47]. Microenvironmental pockets termed niches function as a protective habitat of stem cells and are regulated by a variety of factors, including pH, hypoxia, immune cells, and extracellular matrix [48, 49]. Within this niche, cancer stem cells are able to maintain themselves, and some cells may perhaps even have the ability to acquire stemness; however, the ability to reprogram for the acquisition of stemness has not been fully researched [40, 50].
Cancer Stem Cells
Cancer stem cells , supposedly rare cells that have the self-renewal properties, have the pluripotentiality and result in the production of a hierarchy of progenitor and differentiated cells as normal stem cells have been relatively well examined in hematopoietic malignancies. Leukemia stem cells have been found to be necessary and sufficient for leukemia maintenance. Cancer stem cells have been identified in a variety of leukemias, including acute myeloid leukemia and chronic myeloid leukemia [51–54]. Initial genetic hits have been identified in stem cells associated with hematopoietic tumors [55]. Once stem cells in the lung become malignant, these cells then proliferate, dividing to form one differentiated daughter cell and one daughter stem cell, maintaining the lung cancer stem cell population. Similar findings have been shown with some solid malignancies, including the brain and breast tumors [53, 56–58]. Disseminated or migrating cancer stem cells probably play a causative role in the development of metastatic disease [59]. Studies also show these cancer stem cells to exhibit resistance to chemotherapy and radiotherapy [58]. Studies also propose that the therapeutic stress of chemotherapy and radiotherapy may stimulate cellular plasticity, mediating the conversion of normal cancer cells to cancer stem cells [60, 61]. Lung cancers are often heterogeneous; but it is currently uncertain whether different cancer stem cell clones cause tumor heterogeneity or whether cancer stem cells have the pluripotentiality of normal stem cells [62].
Lung Stem Cells
The presence in adults of normal lung stem cells remains controversial, because lung epithelium is traditionally considered quiescent. Normal lung stem cells are thought to serve in the maintenance of normal lung architecture and reportedly lie in functionally and anatomically distinct sites in the respiratory tract. It is hypothesized that these normal lung stem cells deregulate, leading to the development of disease. These lung stem cells exhibit specific characteristics; for example, proximal airway stem cells have been shown to have higher Keratin 5 promoter activity , and bronchial basal cells have the ability to form heterogeneous spheres in vitro, evidence of self-renewal and multipotent potential [63, 64]. Sox2 has been implicated in the experimental induction of pluripotency and has been linked to basal tracheal epithelium progenitor characteristics. Amplification of chromosomal segment 3q26.33, containing the Sox2 locus, has been found to be associated with pulmonary squamous cell carcinoma, supporting the hypothesis that Sox2 overexpression airway basal stem cells can change them into squamous cell carcinoma cancer stem cells [65, 66].
Lung Cancer Stem Cells
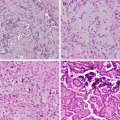
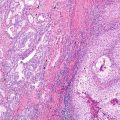
Although the understanding of lung cancer continues to progress, there are many areas for which there is not a complete understanding, but without which the development of successful therapies will remain elusive. As with other solid organ tumors, lung cancers can be hypothesized to contain hierarchically heterogeneous populations of transformed tumor cells with differing levels of differentiation and variably reduced potential for regeneration. Quiescent cells termed cancer stem cells allow the tumor to sustain itself and progress due to their abilities for self-renewal and asymmetric cell division. Cancer stem cells have the capacity for quiescence, indefinite self-renewal, the ability to produce differentiated progeny, and an intrinsic resistance to chemotherapy and radiotherapy. Cancer stem cells provide for tumor growth, resistance to therapy, tumor relapse, and tumor metastasis [42, 67]. Various hypotheses suggest cancer stem cells originate from normal somatic cells, somatic stem cells, and progenitor cells; however, the somatic stem cell hypothesis is most supported [68]. As cancer stem cells arise in a wide variety of tissues, they clearly exhibit uniform characteristics such as the ability to reestablish the primary tumor’s phenotypic heterogeneity after serial transplantation in immunocompromised mice, and the ability to form mammospheres in culture in nonadherent conditions in vitro [51, 69]. There is substantial evidence of several stem cell populations in normal lung that are relatively specific to certain areas of the lung [22, 70–75].
Strongly CK5-immunopositive basal cells within submucosal gland ducts in the mouse trachea have been identified and are considered possible stem cells or progenitor cells involved in regeneration or repair of tracheal epithelium [70]. Club cell secretory protein-expressing cells, also termed CE cells , and basal cells that line mouse bronchi may be stem cells or progenitor cells [74, 76, 77]. The bronchioles in the mouse lung, CE cells, associated with pulmonary neuroendocrine cells, are arranged in small bodies of cells termed neuroepithelial bodies. The CE cells in the bronchioles are pollution resistant, most likely due to cellular deficiency of the drug metabolizing enzyme CYP450 2F2 [75]. The bronchoalveolar duct junction in mice has been shown to contain pollution-resistant CE cells, not associated with neuroepithelial bodies, exhibiting both alveolar epithelia type II cell marker, surfactant protein C, and Club cell secretory protein. These cells probably play a reparative role for the terminal bronchioles, alveolar ducts, and alveoli [73]. These cells were identified as stem cells due to their expression of stem cell surface markers Sca-1 and CD34. Another stem cell niche of “variant” Club cells has been identified arising in the same location [72, 73, 78]. A third possible stem cell population has been identified, differing from the bronchioloalveolar stem cell population noted above by their CD34 immunonegativity and their immunopositivity with Oct-4 and SSEA-1, both embryonic stem cell markers related to self-renewal and pluripotency [79, 80]. Oct-4 positivity suggests the possibility that bronchioloalveolar stem cells arise from Oct-4 positive neonatal lung cells [78, 81]. Homeostatic regulation of bronchioloalveolar stem cell niches has been associated with expression of several tumor suppressor genes [78]. In mouse lung studies, stem cell niches have been identified that maintain epithelial differentiation within the airways. These niches are likely targets for lung cancer initiation and promotion [19, 73, 74]. It is thought that there may be as many as 40 different epithelial, mesenchymal, vascular, and lymphatic endothelial and immune cell lineages in the lung [17, 82]. It is important to remember that “lung cancer” is actually a variety of malignant pulmonary neoplasms that arise from cells that are phenotypically different [17, 19, 26].
Lung Cancer Stem Cell Regulation, Gene Expression, and Cell Surface Markers
There have been some common molecular pathways identified that are important in the development of cancer stem cells. Three embryonic patterning pathways, Notch, Hedgehog, and Wnt, are involved in early events leading to expansion and malignant transformation of normal stem cells in the lung [78, 83]. The Notch pathway is important for development and homeostasis in stem cells and helps stem cells maintain viability by asymmetric cell division. Notch signaling is required for lung development, and elevated Notch ligand and receptor levels have been shown in non-small cell lung cancer cell lines [78, 84]. The Hedgehog pathway is also important in early lung formation, and studies have shown it to be involved in epithelial-mesenchymal interactions controlling the branching of developing lung buds. In adult lungs, Hedgehog signaling is normally identified only at low levels in rare cells located in bronchial epithelium basal layers [78, 85–87]. Persistent activation of the Hedgehog pathway has been shown in small cell lung cancers, but uncommonly in non-small cell lung cancer cell lines [19, 88]. Wnt pathway signaling is also important in early lung development and lung disease [19, 89, 90]. Wnt pathway disruption has been found to be a factor in the development of non-small cell lung cancers [19, 91, 92]. These three pathways offer opportunities for future therapeutic intervention.
There are other genes involved with cancer stem cells, including Oct-4 (also termed Oct-3 and POU5F1), a gene regulated by the Wnt pathway that is involved in the maintenance of stem cell pluripotency. Interestingly, Oct-4 has been shown to be capable of reprogramming committed somatic cells and induce those cells to dedifferentiate and revert to an earlier, more developmentally potent state. Keratinocytes that overexpress Oct-4 have been shown to differentiate into other cell types [19, 93, 94].
There are various cell surface markers that may help identify cancer stem cells [19, 95]. These and future markers are important in helping to identify these stem cell niches and to identify mechanisms that transform normal stem cells into cancer stem cells. CD44 , a transmembrane cell-surface adhesion glycoprotein involved in cell-cell interactions and cell-matrix interactions, linked to chemoresistance and poor prognosis in various malignant neoplasms, is increased in, and correlates with survival in, both non-small cell lung cancer and small cell lung cancer [19, 95–97]. CD133 , also termed prominin-1 , is a glycoprotein found in endothelial cells that has been identified in cancer-initiating stem cells of the brain, pancreas, and colon. As with CD44, CD133 is associated with chemoresistance [98]. Non-small cell lung cancers and small cell lung cancers have been found to have CD133-positive tumor cell subpopulations, showing similarities to a rare CD133-positive population of normal mouse lung cells that undergo significant expansion after naphthalene-induced lung injury [19, 95, 99]. Phosphatase and tensin homologue deleted on chromosome ten (Pten) inactivation in side population cells has been shown to result in spontaneous lung tumors; and studies have shown that activation of mammalian target of rapamycin (mTOR), involved in expression of CD133 in cancer cells, is related to upregulation of stem cells and progenitor cells in Pten conditional deletion models, suggesting that mTOR may be a potential therapeutic target [100–102].
CD117 , also termed c-Kit , is a stem cell factor in neuroendocrine lung tumors that is related to poor prognosis in early-stage non-small cell lung cancers; however, less than one third of patients exhibit CD117 tumor cell positivity [19, 95, 103]. While other solid organ cancers, such as brain and breast cancers, have demonstrated a variety of putative cancer stem cell markers, few other potential cancer stem cell markers have to date been identified in lung cancers. Unfortunately, two cell surface markers found in mouse bronchioloalveolar stem cells have given disappointing results in human studies. Sca-1 does not have a human counterpart, and CD34 does not correlate with putative human non-small cell lung cancer stem cells. Additional studies are necessary to identify and confirm whether each type of lung cancer arises from a single, normal lung stem cell, or whether there are multiple stem cell origins responsible for each cancer’s cellularity [95, 104]. The cell surface markers urokinase plasminogen activator (uPA) and its receptor uPAR, also termed CD87 , have been identified in small cell lung cancer cells that coexpress CD44; however, their contribution to the development and maintenance of a lung cancer stem cell population is not currently known [19, 105].
Lung Cancer Progression
Nonneoplastic tissue exhibits orderly development, with numerous cell types maintaining generations of differentiated progeny cells via epigenetic regulation; however, neoplastic cells exhibit disorganized cell programming, producing populations of heterogeneous tumor cells [106–109]. The niche provides a site of equilibrium between the cancer stem cells and the differentiated cells they produce, allowing for cancer development [110]. One study showed that for breast and prostate cancer, interleukin-6 has been identified as a factor in the transformative reprograming of non-cancer stem cells within the niche to cancer stem cells [110]. Epithelial-to-mesenchymal transition (EMT) has also been considered, along with its role in metastasis, as a method of cancer stem cell population maintenance within the niche [111].
Malignant neoplasms are relatively hypoxic due to their limited vasculature; and hypoxia with low pH reportedly maintains the niche’s ability to maintain cancer stem cell self-renewal via the activation of genes associated with stemness [112]. Studies have shown that relatively acidic pH promotes stemness within the niche and correlates with increased tumor size [112, 113]. Hypoxia also is thought to support the dedifferentiation of cells to become cancer stem cells within the niche [40, 49, 114]. By stimulating the release of vascular endothelial growth factor (VEGF) and angiogenic factors, hypoxia also promotes angiogenesis, which is also promoted by the niche’s low pH [112, 115].
Chemotherapeutic and Radiotherapeutic Resistance and Cancer Relapse
Lung cancer stem cells are quiescent and are therefore relatively resistant to conventional chemotherapy and radiotherapy; their quiescence provides for periods of cancer remission, where residual cancer is undetectable by current imaging methods. Frequently, periods of remission occur following a patient’s receipt of prolonged chemotherapy or radiotherapy . Patient outcomes are frequently poor in this setting [116–118]. Pronounced chemoresistance and radioresistance in these patients, with subsequent tumor spread or tumor relapse , is believed to be a function of the neoplasms’ cancer stem cells and likely promoted by the cancer stem cells’ enhanced DNA damage response [117, 119–121]. Because radiation and common chemotherapies kill cancer cells via DNA damage, generally by the production of double-strand breaks, a cancer cell’s inability to repair the DNA damage kills the cell [122, 123]. Rad51 is a repair gene associated with therapeutic resistance; it catalyzes the search for, and the invasion of, the homologous DNA strand and initiates repair via annealing [124]. Overexpression of Rad51 by cancer stem cells is thought to provide for therapeutic resistance [125]. Studies have shown that Rad51 inhibition promotes cancer stem cell therapeutic resensitization, supporting the hypothesis that cancer stem cells overcome DNA damage via their Rad51 expression-enhanced DNA repair ability [126, 127].
Niche Targeting
Due to lung cancer stem cells’ enhanced therapeutic resistance properties, development of therapeutic approaches targeting these niche’s cancer stem cells is vital. Because the niche enables and maintains stemness, limiting or ending cellular plasticity within the niche could destroy the niche environment supporting the cancer stem cell phenotype. Further, because reduced Rad51 expression within the niche may therapeutically resensitize cancer stem cells, research is focusing on finding therapeutic targets that can destroy cancer stem cells by inhibiting their self-renewal properties, by inducing toxicity to directly destroy them, and by resensitizing the cancer stem cells to conventional therapies [128, 129]. Niche targeting is particularly challenging because of niche plasticity and the ability of cancer cells to be reprogrammed into cancer stem cells. One concept is to maneuver cancer stem cells to differentiate and as such lose their stemness, using bone morphogenetic proteins which are known to initiate differentiation in cancer stem cells. Cancer stem cell differentiation might lead to cancer cells targetable with conventional therapies, thereby reducing or eliminating cancer growth and recurrence [129–131].
References
1.
Aoi T. Biology of lung cancer: genetic mutation, epithelial-mesenchymal transition, and cancer stem cells. Gen Thorac Cardiovasc Surg. 2016;64:517–23.PubMed
2.
Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology. 2014;28:1101–7. 1110.PubMed
3.
Koren A, Motaln H, Cufer T. Lung cancer stem cells: a biological and clinical perspective. Cell Oncol. 2013;36:265–75.
4.
Asselin-Labat ML, Filby CE. Adult lung stem cells and their contribution to lung tumourigenesis. Open Biol. 2012;2:120094.PubMedPubMedCentral
5.
Feron F, Delorme B, Nivet E, Gaillard J, Haupl T, Ringe J, et al. The human nose harbours a niche of olfactory ecto-mesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2009;19(6):853–66.
6.
Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2009;222(2):268–77.
7.
Robin C, Bollerot K, Mendes S, Haak E, Crisan M, Cerisoli F, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5:385–95.PubMedPubMedCentral
8.
Nishikawa SI, Osawa M, Yonetani S, Torikai-Nishikawa S, Freter R. Niche required for inducing quiescent stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:67–71.PubMed
9.
Coura GS, Garcez RC, de Aguiar CB, Alvarez-Silva M, Magini RS, Trentin AG. Human periodontal ligament: a niche of neural crest stem cells. J Periodontal Res. 2008;43:531–6.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree