Regimen
N
(%)
AE (N)
AE (%)
Carboplatin + paclitaxel
140
35.4
12
8.6
Carboplatin + etoposide
82
20.7
3
3.7
Cisplatin + etoposide
38
9.6
4
10.5
Vinorelbine
30
7.6
8
26.7
Cisplatin + UFT
17
4.3
5
29.4
Carboplatin + vinorelbine
10
2.5
0
0
Cisplatin + vinorelbine
9
2.3
2
22.2
Docetaxel
7
1.8
1
14.3
Carboplatin + docetaxel
6
1.5
4
66.7
Cisplatin + docetaxel
6
1.5
1
1.7
Gefitinib
6
1.5
5
83.3
Others
51
10
19.6
Total
396
52
13.1
In our prospective feasibility studies of first-line treatment for lung cancer patients with coexisting IIPs, one (5.6 %) out of the 18 patients who received carboplatin with weekly paclitaxel and one (5.9 %) out of the 17 patients who received carboplatin with etoposide developed chemotherapy-related AE of IIPs [29, 30]. Moreover, the objective response rate (ORR) and progression-free survival (PFS) in these studies were comparable to those observed in previous studies for NSCLC and SCLC patients without ILDs (ORR, 61% and 88%; median PFS, 5.3 months and 5.5 months, respectively). Nevertheless, the extent of overall survival (OS) was poorer for patients without ILDs (median OS, 10.6 months and 8.7 months, respectively). Kenmotsu and colleagues retrospectively analyzed 104 NSCLC patients with ILDs treated by platinum-based chemotherapy. Across all patients, the incidence of first-line chemotherapy-related exacerbation of ILDs was 9 %, while the ORR, PFS, and OS were 38 %, 4.8, and 9.9 months, respectively. Five (8 %) out of the 63 patients treated with carboplatin with paclitaxel developed chemotherapy-related exacerbation of ILDs [31].
In SCLC, Yoshida and colleagues reported that one (1.9 %) out of the 52 SCLC patients who received platinum agents with etoposide developed chemotherapy-related exacerbation of ILDs, and the PFS and OS were 4.5 and 9.4 months, respectively [32]. The combination chemotherapies of carboplatin with paclitaxel for NSCLC and carboplatin with etoposide for SCLC are the most widely used of the established standard regimens for advanced lung cancer without ILDs. Furthermore, both of these regimens are the mostly frequently used for lung cancer patients with chronic ILDs, with comparatively permissible safety. Thus, the combination of carboplatin with paclitaxel and carboplatin with etoposide are currently the most recommended treatment options for lung cancer patients with chronic ILDs. However, other studies have reported high risks with these two regimens, with the incidences of AE in these cases ranging from 16–27 % [33–35].
The knowledge base regarding second-line or subsequent treatment is poorer than that in existence for first-line treatment. Most physicians conclude that survival benefit cannot be commensurate to the risk of AE of ILDs and hesitate in respect to the use of subsequent chemotherapy. Therefore, it is considered that one of the possible reasons for unsatisfactory OS despite comparatively good ORR and PFS is less frequently receive second-line chemotherapy in patients with ILDs compared to those without ILDs. As such, it is also essential for improvement of OS to establish optimal chemotherapy for previously treated lung cancer patients with chronic ILDs.
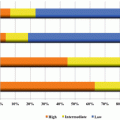
The Diffuse Lung Diseases Research Group retrospectively analyzed chemotherapy-induced AE and the prognosis for lung cancer patients with IIPs who were treated with second-line chemotherapy [the 2012 and 2013 annual report of the Diffuse Lung Diseases Research Group from the Ministry of Health, Labor and Welfare, Japan]. The incidence of AE of ILDs according to each chemotherapy regimen is shown in Table 15.2. The incidence of second-line chemotherapy-related AE was 45 (16.2 %) out of the 278 patients examined, which is comparable to that seen with first-line chemotherapy. Docetaxel (25.9 %) was the most frequently used agent in the context of subsequent chemotherapy. However, docetaxel, pemetrexed, topotecan, and amrubicin, when used as standard agents for previously treated NSCLC and SCLC, lead to chemotherapy-induced AE of ILDs (AE rates, 15.3 %, 28.6 %, 23.1 %, and 33.3 %, respectively). On the other hand, the use of carboplatin with paclitaxel (9.7 %), carboplatin with etoposide (0 %), or S-1 monotherapy (0 %) appeared to confer a low risk in respect of AE of ILDs. These treatments have not been established as standard of care for previously treated NSCLC or SCLC. A major limitation to this retrospective analysis was that there were only a small number of patients who received each regimen. In relation to survival analysis in this study, OS from second-line and first-line treatment were 8.6 and 15.7 months for NSCLC patients and 9.0 and 17.3 months for SCLC patients, respectively. These encouraging results are comparable with previous reports of patients without ILDs, suggesting that second-line treatment may contribute to improving the prognosis of advanced lung cancer patients with ILDs.
Table 15.2
The incidence of acute exacerbation of idiopathic interstitial pneumonias related to each second-line chemotherapy regimen
Regimen | N | (%) | AE (N) | AE (%) |
|---|---|---|---|---|
Docetaxel | 72 | 25.9 | 11 | 15.3 |
Carboplatin + paclitaxel | 31 | 11.1 | 3 | 9.7 |
Carboplatin + etoposide | 15 | 5.4 | 0 | 0 |
Vinorelbine | 24 | 8.6 | 6 | 25 |
Pemetrexed | 21 | 7.6 | 6 | 28.6 |
Amrubicin | 18 | 6.5 | 6 | 33.3 |
Topotecan | 13 | 4.9 | 3 | 23.1 |
S-1 | 14 | 5.3 | 0 | 0 |
EGFR-TKIs | 9 | 3.2 | 4 | 44.4 |
Paclitaxel | 7 | 2.5 | 1 | 14.3 |
Cisplatin + vinorelbine | 6 | 2.2 | 0 | 0 |
Irinotecan | 6 | 2.2 | 0 | 0 |
Others | 42 | 5 | 12.5 | |
Total | 278 | 45 | 16.2 |
15.3.2 Molecular-Targeted Agents
Over the past decade, introduction of molecular-targeted agents has dramatically changed the treatment landscape of NSCLC. Specific inhibitors targeting various driver oncogene mutations have demonstrated a higher response rate and longer PFS than platinum doublet-based cytotoxic chemotherapy. Molecular-targeted agents available to lung cancer patients in Japan include the EGFR-TKIs and anaplastic lymphoma kinase (ALK)-TKIs against EGFR-activating mutations and ALK rearrangements, respectively, and bevacizumab, which is an angiogenesis inhibitor targeting VEGF activity. Adverse events associated with the use of molecular-targeted agents are generally less severe than that experienced with cytotoxic chemotherapy agents. On the other hand, the rare but potentially fatal adverse event, DILD, has been reported in Japanese patients treated with molecular-targeted agents, such as EGFR-TKIs and ALK-TKIs [36–38]. In addition, the incidence of DILD has been reported to be higher in Japanese patients compared to Caucasians.
With regard to lung cancer patients with chronic ILDs, driver oncogene mutations are rare in this population compared to those without ILDs; other defining characteristics of the former group of patients include male gender, current or former smokers, and low frequency of adenocarcinoma histology. Thus, it is predicted that there are few patients with coexisting ILDs for whom molecular-targeted agents can contribute to positive survival effects.
15.3.2.1 EGFR-TKIs
The use of EGFR-TKI is a standard treatment allowing targeting of NSCLCs harboring EGFR active mutations. Currently available EGFR-TKIs in Japan are gefitinib, erlotinib, and afatinib. Three large-scale studies of ILD associated with EGFR-TKI usage have been conducted in patients unselected by EGFR mutations in Japan. These studies suggest that preexisting ILDs and history of smoking were common predictive risk factors (Table 15.3). In a prospective cohort and nested case-control study by Kudoh and colleagues, the cumulative incidence of gefitinib-induced ILD during 12 weeks of treatment was 4.0 %. Gefitinib significantly increased the risk of DILD compared with conventional cytotoxic chemotherapy. These investigators also described that preexisting ILD was an independent risk factor for DILD, regardless of gefitinib or other chemotherapy usage (odds ratio, 4.8–5.6) [17]. With regard to erlotinib, in the post-marketing POLARSTAR surveillance study in Japan, it was reported that 429 (4.3 %) out of the 9907 patients developed ILD (all grade), and the mortality rate due to ILD was 1.5 % [39]. Concurrent/previous ILD and history of smoking were also identified as significant risk factors for developing ILD (odds ratios, 3.19 and 2.23, respectively). Moreover, concomitant honeycombing with interstitial pneumonia was identified as poor prognostic factor for ILD death (odds ratio, 6.67).
Table 15.3
ILDs related to EGFR-TKI usage in Japanese patients
Ando et al.a | Kudoh et al.b [14] | Gemma et al. [39] | |
|---|---|---|---|
Study design | Retrospective | Prospective | Retrospective |
Number of patients | 1976 | 1482 | 9909 |
ILD (any grade)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|