Author
Technology and dose prescription
N
Results
Follow-up
Debus et al. (Debus et al. 1999)
Median 52.4 Gy/1.6–2.0 Gy Margin: 7 mm
10
5-year PFS: 90%
12–72 mos
5-year OS: 100%
No acute toxicities
Saran et al. (Saran et al. 2002)
Median
14
3-year PFS: 87%,
33 mos
50–55 Gy/30–33 fractions
3-year OS: 100%
1 relapse within GTV
Margin: 5–10 mm
Hug et al. (Hug et al. 2002)
Protons
27
Local control
survival
3.3 years
50.4–63.0 CGE/1.8 Gy
Hemispheric
71%
86%
Diencephalon
87%
93%
Margin: no data
Brainstem
60%
60%
Marcus et al. (Marcus et al. 2005)
Stereotactic technique median
81
5/8-year PFS: 82.5%/65%
6.9 years
52.2 Gy/1.8Gy
5/8-year OS: 97.8%/82% 6 local relapses
Margin: 2 mm
All within field
Combs et al. (Combs et al. 2005)
3D conformal RT
15
3/5-year PFS: 92%/72%
97 mos
Med. 52.2 Gy/1.8Gy
5-year OS: 90%
Margin 5 mm
Merchant et al. (2009)
3D conformal RT
78
5/10-year EFS: 87.4%/74.3%
89 mos
Median
5/10-year OS: 98.5%/95.9%
54 Gy/1.8 Gy
13 relapses
Margin: 15 mm
(8/13 within PTV)
(1/13 marginal)
(4/13 CNS metastatic)
Paulino et al. (2013)
IMRT
39
8-year EFS/OS: 78.2 and 93.7%
81 mos
45–60 Gy
7/7 failures in field
5–10 mm
Muller et al. (2013)
Conventional/3D planning
75
Pilocytic astrocytoma
8.4 years
Median 54 Gy/1.8 Gy
5/10-year PFS: 76.5%
Margin 1–2 cm
5/10-year OS: 96.2%
Relapse pattern: N/A
In the only prospective multicentre HIT-LGG 96 trial, 117 patients with pilocytic astrocytoma underwent radiation therapy. The 5- and 10-year progression-free survival rate after external fractionated radiation therapy was 76% with a 10-year overall survival rate of 97%. Disease progression was not influenced by gender, neurofibromatosis type 1 status, tumour location, age or prior chemotherapy (Mueller et al. 2013) (Table 10.1).
10.7.3.1 Response of Tumour to Treatment
Only a few series indicate the response of low-grade glioma to radiotherapy with respect to tumour size and clinical symptoms (Bakardjiev et al. 1996; Fisher et al. 1998). Increase in tumour size after radiotherapy may be seen and often not accompanied by clinical signs and symptoms. This increase in size or pseudoprogression might nevertheless be misleading and misinterpreted as recurrent disease.
10.7.3.2 Radiotherapy Following Chemotherapy
Chemotherapy is associated with a high rate of early progression. In the HIT-LGG 96 trial, the 5- and 10-year progression-free survival rates following the start of treatment were 47 and 44% after a median observation time from diagnosis of 10.4 years (Gnekow et al. 2012). It appears that this subset of patients represents a cohort with biologically more aggressive tumours, and the additional question of whether chemotherapy renders the tumours more radioresistant needs to be considered. In the series of Janss et al., 46 children under the age of 5 years received first-line chemotherapy (Janss et al. 1995). Subsequently, 17 children received radiotherapy because of progressive disease; 7 of 17 children who required radiation after chemotherapy have incurred a third progression and the second progression-free survival was 29% at 10 years. Paulino treated 9 children with IMRT who failed initial chemotherapy and 30 children with radiotherapy as first-line treatment (Paulino et al. 2013). The 8-year progression-free survival rates for children who did and did not receive prior chemotherapy were 50 and 88.4% (p < 0.03). In the prospective HIT-LGG 96 trial, no difference was found regarding the use of previous chemotherapy. The 10-year PFS was 75% in 17 patients having received primary chemotherapy as compared with 77.6% in 58 patients after primary radiotherapy (Mueller et al. 2013).
10.7.3.3 Dose Response Effects
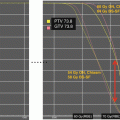
The optimum dose for radiation therapy in childhood low-grade glioma has not been well established. In children, no prospective randomized studies of radiotherapy dose response have been performed. The selection of dose prescriptions is strongly influenced by patient age, extent and site of tumour with a tendency to use a lower dose in younger children with larger tumours (larger treatment portals). The recommended and generally accepted dose prescription ranges between 45 and 54 Gy in 1.8 Gy fractions depending on age at treatment, extent of disease and location of tumour. Retrospective data, however, indicate that a dose of 50.4 Gy is equally effective as 54 Gy. In the HIT-LGG 96 trial, no differences were observed. In the retrospective series from Houston patients receiving 50.4 Gy or below had an 8-year PFS of 73.8% as compared with 90.9% after dose prescriptions of above 50.4 Gy, without statistically significant difference (Paulino et al. 2013) (Table 10.2).
Table 10.2
Radiotherapy dose response and progression-free survival in children and adults with low-grade glioma
Author | N | Total radiotherapy dose | Fraction size | PFS (5 years) | PFS (10 years) | p-value |
|---|---|---|---|---|---|---|
Montgomery et al. (1977) | N/A | Overall | N/A | N/A | ||
7 | ≤42 Gy | 43% | ||||
9 | ≥50 Gy | 100% | ||||
Sung (1982) | N/A | Relapse rate: 11/13 | N/A | N/A | ||
13 | 35–45 Gy | |||||
29 | 50–60 Gy | 8/29 | ||||
Alvord and Lofton (1988) | 52 | >45.0 Gy | N/A | 80% | 65% | N/A |
62 | <45.0 Gy | 65% | 55% | |||
Flickinger et al. (1988) | 12 | >45.0 Gy | Calculation according nominal standard dose | 100% | N/A | P = 0.045 |
12 | <45.0 Gy | 75% | ||||
Kovalic et al. (1990) | 3 | <40.0 Gy | N/A | 0 | 0% | <0.0001 |
30 | >40.0 Gy | 90% | 79% | |||
Garcia et al. (1990) | 8 | <40 Gy | N/A | 4/8 recurred | N/A | N/A |
17 | ≥40 Gy | 2/17 recurred | ||||
Jenkin et al. (1993) | 19 | >50.0Gy | N/A | 88% | 88% | 0.37 |
15 | <50.0 Gy | 72% | 57% | |||
Grabenbauer et al. (2000) | 9 | 44–45 Gy | 1.6–2.0 Gy | 87% | 36% | 0.04 |
16 | 45.1–60 Gy | 90% | 85% | |||
Paulino et al. (2013) | 5 years | 8 years | 0.37 | |||
29 | ≤50.4 Gy | 1.8 Gy | 73.8% | 95.2% | ||
10 | >50.4 Gy | 90.9% | 88.9% | |||
Muller et al. (2013) | 13 | ≤50.4 Gy | 1.8Gy | 77% | 77% | 0.941 |
52 | >50.4 Gy | 77% | 77% |
10.7.3.4 Proton Therapy
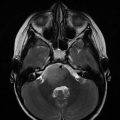
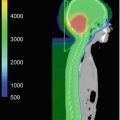
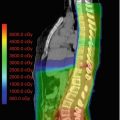
The major advantage of proton therapy over conventional radiation techniques is the high degree of dose conformity around the tumour that can be achieved as well as reduction of low dose radiation region to surrounding brain and other critical structures. The working group of Loma Linda treated 27 paediatric patients with progressive or recurrent gliomas at various sites (Hug et al. 2002). Target doses were between 50.4 and 63.0 Cobalt Gray Equivalent (CGE) at 1.8 CGE per fraction. At a mean follow-up period of 3.3 years, six patients experienced local failure and four died of disease. Dennis et al. compared proton therapy plans with IMRT plan for dosimetric differences to surrounding tissue. Proton therapy effectively reduces the dose to surrounding normal tissues as compared with IMRT. The authors concluded that the benefit of proton therapy over IMRT may be more substantial in patients with tumours in proximity to critical structures. (Dennis et al. 2013). In a series from Boston, 32 children received a median radiation dose of 52.2 CGE. The 6- and 8-year rates of progression-free survival were 89.7% and 82.8%, respectively, with an 8-year overall survival of 100%. There were no significant declines in full-scale intelligence quotient. However, some significant decline in neurocognitive outcomes for young children (<7 years) and those with significant dose to the left temporal lobe/hippocampus were observed. The incidence of endocrinopathy correlated with a mean dose of ≥40 CGE to the hypothalamus, pituitary or optic chiasm. Stabilization or improvement of visual acuity was achieved in 83.3% of patients (Greenberger et al. 2014).
10.7.3.5 Brachytherapy
The purpose of interstitial brachytherapy is to deliver a focal necrotizing radiation dose within the tumour while sparing normal surrounding tissue. There is a steep dose gradient at the periphery thereby leaving a high cumulative dose around the implanted radioactive seeds, most commonly Iodine-125. Two major German series investigated the temporary iodine and permanent iodine implantation in 94 and 147 patients, respectively. Progression-free and overall survival were excellent (Table 10.3). In the series of Ruge, the neurological status at last follow-up was improved in 57.8% and unchanged in 23% of patients. Fifteen (11.3%) of 132 patients had no symptoms and 4 (3%) presented with treatment induced permanent deterioration of their neurological status (Ruge et al. 2011). It seems that small, circumscribed tumours with a diameter of less than 4 cm in locations other than the optic nerve and chiasm are preferred cases for interstitial brachytherapy. Prospective data on neurocognitive function are not available.
Table 10.3
Outcome after interstitial brachytherapy in childhood low-grade glioma using modern treatment technologies
Author | Technique | Tumour size | Patients | Outcome |
|---|---|---|---|---|
Herrera et al. (2007) | Temporary I- | <4 cm | n = 12 | OS: 83.3% |
125 seeds | age range: 8–17 yrs | |||
60 Gy | (median: 8.7 yrs) | |||
Pilocytic astrocytoma only | ||||
Peraud et al. (2007) | Temporary I- | <4 cm | n = 11 | Initial volume: 14.9 mL |
125 seeds | ||||
54 Gy | age: 11 mos to 16 yrs | CR: n = 4 | ||
PR: n = 7 | ||||
OS: 100% | ||||
(median: 6.8 yrs) | ||||
Grade I: n = 6 Grade | ||||
II, n = 5 | ||||
Korinthenberg et al. (2011) | Temporary I- | <5 cm, spherical shape | n = 94 | 5- and 10-yr OS: |
125 seeds | age range: 1–19 yrs | 97 and 92% | ||
Median dose 60 Gy | median age: 9 yrs | |||
Mean treatment time 28d | Grade I and II | |||
Ruge et al. (2011) | Permanent I- | <4–5 cm, | n = 147 | 5-, 10- and 15-yr PFS: |
125 seeds. Median 50–65 Gy | Demarcated lesions | age: 1.2–19.9 yrs, | ||
Median:10.7 yrs Grade I and II | 92, 74 and 67% | |||
5-, 10- and 15-yr OS: | ||||
98, 93 and 82% | ||||
Mueller et al. (2013) | Permanent and temporary I- | n = 42 | 5- and 10-yr PFS: 65 and 58% | |
125 seeds | 5- and 10-yr OS: 97 and 97% |
10.7.3.6 Radiosurgery
Clinical data on radiosurgery for paediatric low-grade glioma are scarce. Weintraub et al. reported on 15 patients with pilocytic astrocytomas. The median maximum dose was 36 Gy while the median marginal dose was 15 Gy (Weintraub et al. 2012). For the total cohort, 83% of patients were progression free at a median of 74 months. No case of radiation-induced necrosis was reported. Tumour volume <2 mL was significantly associated with an improved progression-free survival. Kano et al. treated 50 patients with pilocytic astrocytoma. Median age was 10.5 years with a median marginal dose of 14.5 Gy. The 1-, 3- and 5-year progression-free survival rates were 91.7%, 82.8% and 70.8%, respectively. A target volume less than 8 mL was significantly associated with a better progression-free survival. Five of 20 patients experienced adverse radiation effects with necrosis and symptomatic oedema requiring supportive treatment (Kano et al. 2009). The Karolinska working group treated 16 patients with radiosurgery for residual tumour after surgery. In 85%, a moderate tumour volume reduction was observed after radiosurgery. Adverse radiation effects were seen in 25% (Boëthius et al. 2002). Hadjipanayis treated 49 patients with radiosurgery, with 37 patients harbouring a pilocytic astrocytoma. Tumour volumes ranged between 0.42 and 45.1 mL. The median dose ranged between 9.6 and 22.5 Gy. Of the 49 patients, 45 were alive after a median follow-up of 32 months post-radiosurgery (Hadjipanayis et al. 2003). In the series of Heppner (n = 49), the median clinical progression-free survival was 44 months while the median radiological progression-free survival was 37 months. The 5-year clinical progression-free survival was 49% (Heppner et al. 2005).
The major criticisms and shortcomings of the retrospective series are limited patient numbers, short follow-up intervals, and cohorts including high-grade glioma and brainstem glioma, adults and children without specifically addressing pilocytic astrocytomas or Grade II tumours in childhood.
10.7.3.7 Radiotherapy in Grade II Astrocytoma
Grade II astrocytomas in children are rare. In the European childhood low-grade glioma series of Stokland and Gnekow, 5.9 and 4.5% out of a total of 1940 cases, respectively, presented as Grade II diffuse astrocytoma (Gnekow et al. 2012; Stokland et al. 2010). According to genomic and transcriptomic analyses, paediatric astrocytoma Grade II carry distinct genetic signatures (Jones et al. 2011). Mutations of IDH 1/2 and TP 53 and MGMT methylation are common in adult but rare in Grade II astrocytomas (Jones et al. 2011). Data on survival for protoplasmic and fibrillary astrocytomas showed a 10-year survival rate of 81.2% for age <20 years (n = 100) vs. 39.2% for age 20–44 years (n = 221) ((Jones et al. 2011), CBTRUS). The outcomes of Grade I and II tumours have been conflicting, with some having better results with Grade I while others having equivalent results (Table 10.4). Stokland et al. noted a significantly better PFS for Grade I tumours (5-year PFS, 72% for pilocytic astrocytoma as compared with 43% for Grade II tumours). Fouladi observed a 5-year PFS of 68% for Grade I tumours vs. 52% in Grade II tumours (Fouladi et al. 2003). For overall survival, the studies by Fisher et al. and Fouladi et al. saw a significantly superior outcome for Grade I tumours (95% in both series at 5 years as compared with 48% and 64%, respectively, for Grade II tumours). The report by Packer et al. did not see a significant difference (Packer et al. 1997). The large series of Gnekow et al. observed a 10-year EFS for 59 patients with Grade II tumours of 40% as compared with 50% in 671 patients with pilocytic astrocytoma histology. While the EFS were statistically significantly different, the overall survival did not differ significantly (94% for pilocytic astrocytoma and 87% for diffuse astrocytoma) (Gnekow et al. 2012). The extent of resection is an important prognostic factor for Grade II astrocytomas. In the series by Mishra et al., the 5-year PFS rates were 79% for patients treated with gross tumour resection (GTR), 60% for those with subtotal tumour resection (STR) and 46% for those with biopsy alone. The corresponding 5-year overall survival rates were 100% after GTR, 88% for those with STR and 89% for biopsy alone (Mishra et al. 2006). The cohort was analysed for the impact of early or delayed radiation therapy irrespective of extent of resection. The 5- and 10-year PFS rates were 50 and 43% for those not receiving early radiation therapy as compared with 61 and 43% for the early radiation therapy group. The 5-year and 10-year overall survival rates were 97% and 92%, respectively, for those receiving no radiation at diagnosis as compared with 84 and 74% for those receiving radiation therapy upon progression (Mishra et al. 2006). An analysis of 24 patients that were included in the German HIT-LGG 96 and 2004 outcome was poor after salvage radiotherapy for progressive disease (Mueller et al. 2012). With a median follow-up of 2.0 years, 11 patients (45.8%) experienced progression and 8 patients (33.3%) died. The 3- and 5-year progression-free survival rates were 56% and 28%, respectively, and the 5-year overall survival was only 63%.
Table 10.4




Outcome of childhood low-grade glioma according to grade
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree