Low anterior resection (LAR), with its numerous technical modifications, is one of the most commonly performed operations for rectal cancer. In the past, patients with locally advanced distal rectal cancer were most frequently treated by abdominoperineal resection and permanent colostomy. However, over the past two decades and with improved understanding of tumor biology and refinement in technique, use of LAR to treat rectal cancer has increased substantially. Yet, despite the significant increase in LAR and sphincter preservation, patients in many areas of the country have little access to these techniques and continue to commonly be treated with abdominoperineal resection. This article examines the surgeon’s unique and critical role in the pretreatment evaluation and decisions leading to choice of surgical therapy for locally invasive distal rectal cancer. In particular, the authors focus on technical aspects to preserve the anal sphincter, and review methods to optimize functional outcomes in the setting of low pelvic anastomosis.
Workup and evaluation
A thorough and comprehensive evaluation of the patient with rectal cancer is critical in order to select optimal surgical therapy. The evaluation includes an assessment of patient suitability for surgery as well as clinical tumor staging. Proctectomy is a difficult procedure for even the fittest of patients, so every effort should be made to optimize patient comorbidities before surgery. Thus, a complete clinical assessment is essential to gauge comorbidities and define operative risk as well as to explore the patient’s goals in treatment. An assessment of sphincter function and preexisting issues related to fecal incontinence or prior sphincter injury is also essential in assessing suitability for sphincter preservation.
The patient with rectal cancer should undergo a thorough evaluation of the colon and rectum with colonoscopy to assess the primary tumor and to identify and remove synchronous polyps or to localize and biopsy synchronous cancers. When possible, the distal border of the tumor is tattooed if chemoradiation is to be provided preoperatively, because determining the distal extent of the tumor is often difficult following neoadjuvant therapy. Given the high rates of complete pathologic response, it is not uncommon for a patient to have no visible lesion at the time of proctectomy, rendering the preoperative tattoo critical. Colonoscopy is important for identification of synchronous cancers or other large unresectable polyps, which may alter treatment plans. If colonoscopy is incomplete, imaging with a double-contrast barium enema or virtual colonoscopy is a suitable alternative. If preoperative surveillance of the proximal colon is not possible, intraoperative colonoscopy can be considered.
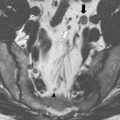
Accurate local and regional staging with radiographic imaging is also essential to select the most appropriate treatment regimen for patients with rectal cancer. Local staging begins with rectal examination and proctoscopy or flexible sigmoidoscopy to determine the location of the tumor in relationship to the anal sphincter mechanism. Imaging is a critical component of the workup for rectal cancer, not only to assess T and N stage but also to provide information regarding contiguous spread from the primary rectal cancer to adjacent nearby structures such as the sphincter mechanism, lateral pelvic sidewalls, prostate, seminal vesicles, vagina, bladder, and sacrum. The precise extent of such local invasion can be assessed by several modalities such as magnetic resonance imaging (MRI), endorectal ultrasonography, or pelvic computed tomography (CT) scanning. MRI is one of the more accurate methods to assess depth of rectal cancer invasion, thereby accurately predicting potentially involved circumferential margins as well as enlarged lymph nodes. Although endorectal ultrasonography characterizes depth of penetration (T stage) and is useful in assessing involvement of the anterior structures such as the vagina or prostate/seminal vesicles, this technique is not as effective in assessing lateral extrarectal spread. MRI is also well suited for identifying poor prognostic features such as extramural spread greater than 5 mm, extramural venous invasion, nodal involvement, and peritoneal infiltration. Given the importance of accurate local staging, MRI has increasingly become the preferred diagnostic imaging study for rectal cancer.
In addition to local staging, evaluation for distant disease is similarly critical, because detection of metastases may completely change the approach to a rectal cancer. The lungs and liver are the most common organs to harbor distant metastases. Patients with rectal cancer should undergo evaluation with either chest radiography or CT to exclude pulmonary metastases. Abdominal CT or right upper quadrant MRI is also recommended for evaluation of liver metastases. Although metastases can occur to other organs, additional imaging is recommended only if symptoms or findings on physical examination raise suspicion of other sites of spread. At this time the role of other imaging modalities, such as positron emission tomography scanning, in the primary evaluation of colorectal cancers is not yet completely understood.
Team approach (neoadjuvant therapy)
There are several treatment strategies available to the patient with rectal cancer, which depend on the preoperative evaluation outlined earlier. Regardless of stage, most proximal rectal cancers are intraperitoneal and biologically mimic the clinical behavior of colon cancers. Conversely, the treatment of mid to distal, locally advanced rectal cancers is variable, generally relying on multimodal protocols that are formulated based on tumor stage and other factors delineated during the preoperative assessment. The aim of this article is to specifically focus on locally advanced disease, so the authors do not include here a discussion of surgical approaches to superficial tumors of the rectum amenable to local excision or proximal cancers of the rectum. Local excision is a viable option for early-stage tumors of the rectum, and these approaches are outlined in more detail in other articles.
Surgical approaches to locally invasive distal rectal cancer are complex because these tumors have a high risk for positive circumferential (radial) or distal margins leading to a high risk for locoregional recurrence. Thus, neoadjuvant chemoradiotherapy is often recommended to patients with locally advanced mid to distal rectal cancer, because it is associated with decreased local recurrence when compared with postoperative therapy. In a large prospective, multicenter, randomized study, local recurrence was significantly lower and tumor downstaging improved in the preoperative chemoradiotherapy group as compared with the postoperative adjuvant therapy group. Despite equivalent 5-year disease-free survival and overall survival rates, the decreased local recurrence rate in this trial has led the National Comprehensive Cancer Network to recommend consideration for preoperative chemoradiation (long course) for all patients with T3 or T4 rectal cancers. Advantages of neoadjuvant chemoradiotherapy include a high rate of complete pathologic response, lower proportion of positive circumferential margins, and a higher likelihood of sphincter-sparing surgery.
Team approach (neoadjuvant therapy)
There are several treatment strategies available to the patient with rectal cancer, which depend on the preoperative evaluation outlined earlier. Regardless of stage, most proximal rectal cancers are intraperitoneal and biologically mimic the clinical behavior of colon cancers. Conversely, the treatment of mid to distal, locally advanced rectal cancers is variable, generally relying on multimodal protocols that are formulated based on tumor stage and other factors delineated during the preoperative assessment. The aim of this article is to specifically focus on locally advanced disease, so the authors do not include here a discussion of surgical approaches to superficial tumors of the rectum amenable to local excision or proximal cancers of the rectum. Local excision is a viable option for early-stage tumors of the rectum, and these approaches are outlined in more detail in other articles.
Surgical approaches to locally invasive distal rectal cancer are complex because these tumors have a high risk for positive circumferential (radial) or distal margins leading to a high risk for locoregional recurrence. Thus, neoadjuvant chemoradiotherapy is often recommended to patients with locally advanced mid to distal rectal cancer, because it is associated with decreased local recurrence when compared with postoperative therapy. In a large prospective, multicenter, randomized study, local recurrence was significantly lower and tumor downstaging improved in the preoperative chemoradiotherapy group as compared with the postoperative adjuvant therapy group. Despite equivalent 5-year disease-free survival and overall survival rates, the decreased local recurrence rate in this trial has led the National Comprehensive Cancer Network to recommend consideration for preoperative chemoradiation (long course) for all patients with T3 or T4 rectal cancers. Advantages of neoadjuvant chemoradiotherapy include a high rate of complete pathologic response, lower proportion of positive circumferential margins, and a higher likelihood of sphincter-sparing surgery.
Surgical technique
Patients with locally invasive nonmetastatic rectal cancer are generally best treated by a radical resection, a form of either low anterior resection (LAR) or abdominoperineal resection (APR). Anterior resection may be further subdivided into high, low, and ultralow techniques. A high anterior resection generally involves partial proctectomy with intraperitoneal anastomosis, whereas a low resection includes complete mobilization of the rectum and an extraperitoneal anastomosis. Ultralow resection refers to transaction of the rectum just above the pelvic floor with an anastomosis at or just above the top of the puborectalis muscle. Regardless of whether the resection is high or low, the tumor itself with satisfactory proximal, distal, and radial margins must be excised. The decision regarding reestablishment of gastrointestinal tract continuity is then dictated by the conditions after tumor removal as well as other key details obtained during workup.
Initial Steps and Preparation
The patient and bowel is prepared as per the surgeon’s preferences. Despite the data demonstrating similar patient outcomes with or without mechanical bowel preparation, our practice is to provide a bowel preparation for all patients with potential for low anastomosis and diverting stoma. Diverting a low anastomosis with a column of feces in the remnant colon seems illogical and thus it is preferred to mechanically cleanse all of these patients. In addition, all patients with distal rectal tumors should have consideration for preoperative stoma marking by a qualified enterostomal therapist. Patient education as well as an understanding of optimal stoma placement is a critical component of the preoperative evaluation as well as the postoperative course. Ureteral stents are also considered for large, bulky, upper tumors or inflammatory lesions.
In the operating room, the patient is placed in the lithotomy position and a bladder catheter is introduced. Before incision, a digital rectal examination and rigid proctoscopy are performed to empty the rectum and reassess the location of the rectal cancer. During examination under anesthesia, anal sphincter involvement is assessed as well as the response of the tumor to chemoradiation. As previously described in the preoperative evaluation section, to facilitate tumor localization the distal border of the tumor is tattooed before initiating chemoradiation to facilitate tumor identification in the operating suite. In addition, prior to incision the distal rectum is washed with either hypotonic solution or a Betadine solution to reduce the potential for tumor shedding.
It is critical that appropriate instruments and other equipment are available for the optimal conduct of the operation. First, headlights and/or lighted retractors are prepared for visualization deep in the pelvis. Self-retaining retractors are often necessary, as are long and deep retractors such as the St Mark retractor and the Wylie renal vein retractors. In addition to long retractors, the surgeon should have access to long instruments and cautery extenders. Most importantly, the operating surgeon will find that the assistance of a second surgeon is critical during the deep pelvic dissection.
Mobilization
After entry into the abdomen, a thorough exploration is performed to exclude metastases or other potential anatomic variations. The small bowel is then packed into the upper abdomen and the patient is placed in Trendelenburg position. The dissection begins along the line of Toldt as the peritoneum is incised along the left side of the sigmoid and descending colon as far caudad as the splenic flexure. The left ureter and gonadal vessels are identified early and preserved. The colon mobilization extends proximally to the transverse colon as adhesions to the spleen are divided and the splenic flexure is mobilized inferiorly.
Pedicle Ligation
The inferior mesenteric artery is best exposed by retracting the rectosigmoid anteriorly and to the left. The vascular pedicle can then be taken either proximally, that is, high ligation of the inferior mesenteric artery as it branches from the aorta, or more distally, that is, low ligation of the artery just distal to the left colic artery. At present, there is not enough evidence to encourage use of one technique over the other. However, it has been found that for an anastomosis to the mid or distal rectum, division and ligation of inferior mesenteric vein is often critical to assure adequate mobility of the descending colon. Following ligation of the vascular pedicle, it is most convenient to divide the colon with a linear stapler.
Total Mesorectal Excision
The standard approach for low rectal cancers involves total mesorectal excision (TME). This technique involves dissection along the areolar plane between the visceral fascia of the mesorectum and the parietal fascia of the pelvic walls. Proper TME removes the intact mesorectum with all of the lymph nodes and the rectum en bloc. For more proximal rectal cancers, a partial mesorectal excision can be performed with a distal margin of 3 to 5 cm. To perform the procedure, the rectosigmoid is retracted anteriorly and inferiorly toward the pubis to expose the avascular plane posterior to the rectum. Sharp incision of this avascular plane with proper rectosigmoid traction permits air to enter the areolar tissues posteriorly and lateral to the rectum. During the retrorectal portion of the mesorectal dissection, the hypogastric nerves are identified at the sacral promontory with an aim toward autonomic nerve preservation. The presacral (Waldeyer) fascia is then divided under direct vision as the dissection proceeds distally to the level of the coccyx. The rectum is mobilized in a posterior-to-lateral direction, with care taken to maintain the integrity of the endopelvic fascial envelope encasing the bilobed mesorectum.
To expose the anterior dissection plane, the angle of the Trendelenburg position is reduced or the patient can even be placed in reverse Trendelenburg. The dissection proceeds in an anterolateral plane by opening the cul-de-sac. Deep pelvic retractors are used to protect the seminal vesicles and prostate in men or the vagina in women. The surgeon then encounters Denonvilliers’ fascia in the midline anteriorly. In Heald’s classic description of TME, Denonvilliers’ fascia is considered the most anterior limit of the mesorectum and is thus removed with the specimen. Denonvilliers’ fascia is similarly excised for circumferential and anterior rectal tumors to ensure a negative circumferential margin. For posterior tumors, the visceral fascia propria of the rectum is followed and the parietal Denonvilliers’ fascia is spared to minimize risk of injury to the nearby periprostatic pelvic nerves.
The importance of the circumferential resection margin (CRM) on oncologic outcome has gained considerable attention. An involved CRM is associated with increased likelihood of local recurrence and shortened survival. In fact, studies reveal that local recurrence occurred in 22% of patients with a positive CRM but only 5% if the CRM was negative for cancer. Much of the focus on circumferential margins has come from a better understanding of surgical technique and in particular the TME technique, which has led to improvements in oncologic outcomes. Workshops have been held in various European nations for surgeons to acquire the skills needed to perform TME and for pathologists to learn how to properly assess the rectal cancer specimen, including the mesorectum, as described by Quirke and colleagues.
Distal Margin
The choice between sphincter-sparing and APR must be based on assessment of the adequacy of the distal and circumferential margins after complete rectal mobilization. In the past, a distal margin of 5 cm was considered an oncologic necessity, leading to sphincter sacrifice in most cases of distal rectal cancer. However, an evolved understanding of the biology of tumor spread has allowed modification of traditional surgical techniques. It is now known that distal intramural spread of tumor is rare, as is distal lymphatic spread below the gross extent of the tumor. With this knowledge, attempts to decrease the acceptable distal margin and preserve gastrointestinal continuity without sacrificing cancer cure have gained traction.
Because all radical resections for rectal cancer should rely on the same proximal, lateral, and radial mobilization, the decision to perform an anastomosis is based on the ability to obtain clear margins as well as a tension-free, well-vascularized anastomosis. Patient preferences to avoid a permanent colostomy, and adequate preoperative comprehension of altered postoperative bowel function must be taken into account, but ultimately oncologic principles should not be compromised. Occasionally, circumstances arise in which the cancer is larger than anticipated or is extending into tissues outside the boundary of the usual TME dissection. In such cases, a more extensive operation than was originally planned is appropriate to provide curative intent. In these circumstances, performance of an anastomosis may seem technically difficult. Similarly, when the dissection must continue into the anal mucosa or internal sphincter, the ability to reconstruct with an anastomosis may also seem challenging. In these situations, intersphincteric dissection and ultralow anastomosis has been described as a sphincter-preserving alternative to APR for cancers within 5 cm of the anal verge. For those tumors with partial invasion into the upper anal canal, at least a portion of the internal sphincter muscle must be removed to improve the radial and/or distal resection margin. Schiessel and colleagues recently updated a growing experience combining TME and autonomic nerve preservation with a total or partial intersphincteric resection at the intersphincteric groove. When the tumor invades into the external sphincter or levator ani muscle, sphincter preservation with anastomosis is generally contraindicated, although some investigators have described partial external sphincter excision as well. At this time, long-term functional and oncologic results are lacking for this type of radical resection.
Other options for sphincter preservation include coloanal anastomosis as described by Parks and Percy. In this operative technique a pull-through type of anastomosis is performed between the sigmoid or descending colon and the distal rectum or anal canal through the anus. Despite a paucity of data, Parks and Percy advocated for mucosectomy with direct anastomosis that unfortunately leads to mucosectomized anal wall that is nonvisible for surveillance endoscopically. In the original description, 76 patients underwent this operation with recurrence and survival rates comparable to historical controls of patients with similar tumors treated by more traditional operations. All patients had a temporary loop colostomy to protect the anastomosis and none of the patients received radiation therapy. Unfortunately, functional abnormalities were common, as patients described both excess stool frequency and irregularity. There has not been a randomized prospective study regarding the desirability of routine mucosectomy with coloanal pull-through procedures.
The aim in reconstructing a patient following anterior resection is to obtain a 1-cm distal margin and a clean circumferential margin by staying within the mesorectal fascia. Functional outcomes are probably better with preservation of the internal sphincter and avoidance of mucosectomy, but adequate tumor clearance (both intramural and mesorectal) is absolutely critical before an anastomosis can be considered. If clearance is adequate and anal sphincter function appropriate, the surgeon may consider anastomosis. At the time of surgery, options for handling the distal rectum or upper anus include right-angle clamps or staplers. If an open purse-string suture is to be placed in the distal rectal cuff, a right-angle clamp is placed distally on the anorectum and the specimen is divided between 2 clamps. Alternatively, if a double-stapled reconstruction is planned instead, a transverse anastomosis stapler is placed distally. The stapler is fired and the rectum divided, leaving a closed rectal cuff for subsequent anastomosis. The surgeon should then examine the resected specimen to assess the radial and distal margins and evaluate the integrity of the mesorectal and rectal dissection. If the margins are inadequate, the mesorectum has been violated, or perforation of the rectum has occurred, local recurrence is a major concern. The treatment plan may have to be altered to reduce the risk of recurrence.
Anastomosis
Following tumor extirpation, consideration is given to creating an anastomosis that is nonporous while closely approximating the function of the normal rectum. Traditionally, the straight end-to-end anastomosis has been the default anastomotic method following radical proctectomy, but postoperative function is considered poor especially in the setting of a very low anastomosis. Thus, several other techniques have been developed including the side-to-end (Baker technique), colon J-pouch, and coloplasty. Although indications for use of one anastomosis over another are lacking, each anastomotic technique has supporters while particular patient characteristics might sway the surgeon to choose one particular type. In addition, all of the anastomotic techniques can be hand sewn or stapled, depending on the surgeon’s preference as well as the oncologic need for mucosectomy.
End-to-end
The straight end-to-end colorectal anastomosis is the traditional choice after radical proctectomy for cancers of the rectum. The technique can be stapled or hand sewn but with improvements in overall technique and staplers in particular, the anastomosis became easier to perform with any of the circular staplers now available. The advantages of the end-to-end anastomosis are ease of construction and ability to mate the 2 ends with minimal tension or excessive mobilization. However, an increased capacity to easily establish the anastomosis lower and lower in the pelvis has led to 2 problems: increased incidence of anastomotic leaks and anterior resection syndrome, that is, bowel dysfunction that is characterized by excessive passing of stool, clustering of bowel movements, and incontinence. These issues have led many surgeons to consider alternative methods for low pelvic anastomosis such as the side-to-end anastomosis, coloplasty, and colon J-pouch procedures.
Side-to-end
One of the earliest reports of the side-to-end (Baker) anastomosis was published in 1950 by Baker. This anastomotic method was originally described as a “low end to side rectosigmoid anastomosis”; however, with the advent of staplers, surgeons began to use the technique at lower and lower levels in the pelvis. In addition, as the functional consequences of end-to-end anastomosis became evident, the Baker anastomosis became an alternative to the more technically complex colon J-pouch or coloplasty procedure.
The side-to-end procedure is performed with either hand-sewn techniques or the circular stapler. In the very low anastomosis, however, the authors prefer to staple this reconstruction. The anastomosis is performed by placing the anvil of the stapler into the divided distal end of the descending colon. Then, the trocar of the anvil is gently brought out of the antimesenteric side of the colon at a length of 4 to 5 cm from the cut end of the distal colon (proximal bowel). Although some surgeons find it important to place a purse-string suture at the point in which the trocar pierces the bowel wall, this is not necessary. The open end of the descending colon is then closed with either a linear stapler or suture sealed. Lastly, the circular stapler is brought out of the top of the acute Hartmann to anastomose the side of the colon to the end of the anus or rectum.
Colon J-pouch
The colon J-pouch was described separately by Lazorthes and colleagues and Parc and colleagues as a reconstruction modification that reduces the severity of the anterior resection syndrome. Thus, the pouch was developed to improve the bowel function results of patients with low pelvic anastomoses. Although functional improvements were initially believed to be caused by increased storage capacity for stool, it has become more accepted that the functional advantage of the colon J-pouch is not a result of its reservoir capacity but a change in colonic motility. As with the other techniques, this reconstruction method can similarly be performed with hand-sewn or stapled techniques.
The colon J-pouch construction requires several technical components to achieve a functional pouch. In addition to an adequate sphincter mechanism and healthy proximal bowel, the intestine must be mobile enough and the pelvis must be wide enough so that the pouch reaches the lower pelvis. Thus, most often the descending colon must be completely mobilized as well as freeing the distal transverse colon and splenic flexure from its attachments. Often, the left colic artery and inferior mesenteric vein must be ligated to provide adequate length. After adequate mobilization, the descending colon is folded into a J configuration with the efferent limb of the J-pouch no larger than 5 to 6 cm, as efferent limb lengths are associated with difficulty in evacuation. The pouch is constructed by inserting a linear cutting stapler through an apical colotomy and approximating the antimesenteric surfaces. The linear staple line is then inspected for bleeding and a decision is made as to the method of construction, either hand sewn or stapled. If a circular stapler technique is chosen, the anvil of the circular stapler is placed into the prior colotomy and secured in place with a purse-string suture. The circular stapler is then inserted gently through the acute Hartmann and an end-to-pouch anastomosis constructed between the J-pouch and anus. If the decision is to hand sew, then the colotomy at the apex of the J-pouch is left open and brought down to the top of the anus. A hand-sewn one-layer anastomosis can then be performed from below with proper retraction via the transanal route.
Coloplasty
The coloplasty technique was developed in response to difficulties related to the suitability of some patients for colon J-pouches. The coloplasty pouch is a simple technique that permits a tension-free anastomosis without significant alteration of colon anatomy. This technique is particularly advantageous in patients with a thick mesocolon and/or a narrow pelvis who are poor candidates for J-pouch reconstruction. This commonly encountered scenario was identified in 25% of patients in a study in which pelvic anatomy or colon thickness rendered them unsuitable candidates for colonic J-pouch.
The coloplasty procedure is similar to a pyloroplasty, in which a colotomy is performed with an 8- to 10-cm long antimesenteric incision at a point 5 to 6 cm from the divided end of the descending colon. If the decision is to staple the anastomosis, the anvil of the circular stapler is placed into the colotomy and brought out from the divided end of the colon before closure of the coloplasty. The colotomy is closed in a transverse direction, perpendicular to the antimesenteric border, with either absorbable sutures or a linear stapler. An end-to-end anastomosis is then performed with the circular stapler.
Which Anastomosis and When?
The anterior resection syndrome is commonly experienced following LAR and, as discussed earlier, is characterized by frequent bowel movements, fecal urgency, stool fragmentation, incontinence, or a combination of all of these symptoms. Poor gastrointestinal function was the impetus for the development of new pelvic reconstruction techniques that increase neorectal capacity and improve function. However, the functional advantages of these new reconstruction methods, particularly the colon J-pouch, were most discernible early on after operation. More recently, a randomized controlled trial comparing functional outcomes of coloplasty, colon J-pouch, or a straight anastomosis revealed significant and lasting benefits for the colon J-pouch as compared with the straight anastomosis or coloplasty reconstruction.
The end-to-end anastomosis is preferred for reconstruction proximal to 7 to 8 cm, as no studies have established a benefit to the colon J-pouch at levels proximal to 8 cm However, The colon J-pouch is preferred for low anastomoses and generally a small pouch, that is, 5 or 6 cm in length rather than 10 cm, is constructed. For particularly low anastomoses, the colon J-pouch reconstruction is the anastomosis of choice, as data reveal that the greatest functional improvements are observed in patients with a colon J-pouch anastomosis 4 cm or less from the anal verge. In addition to the previously described randomized, controlled trial that demonstrated the long-term functional superiority of colon J-pouch, the long-term functional benefits of colon J-pouch were reinforced in a meta-analysis evaluating bowel function outcomes.
The authors like the side-to-end anastomosis for low pelvic anastomosis whereby the colon J-pouch is technically difficult or in which the side-to-end anastomosis is anatomically easier to construct. Small studies have demonstrated equivalency of the side-to-end anastomosis and the colon J-pouch 2 years after LAR. These data reveal no significant difference in surgical outcome between colon J-pouch and side-to-end anastomosis when the outcomes of blood loss, hospital length of stay postoperative complications, reoperations, or functional results are analyzed. However, this study did favor the colon J-pouch in one category: at 6 months, the ability to evacuate the bowel in less than 15 minutes was worse among patients with side-to-end anastomosis. Unfortunately, comparative data are lacking and given the technical ease of side-to-end anastomosis, this procedure has become an excellent alternative to pouch reconstruction.
The coloplasty was also developed to overcome the difficulty of the pouch reconstruction in a patient with a narrow pelvis and thick mesentery. However, the functional results have been mixed, and other data reveal an increased incidence of anastomotic leak after coloplasty reconstruction as compared with colon J-pouch reconstruction. In the meta-analysis detailed earlier, the authors concluded that further study is necessary to determine the role of coloplasty in coloanal anastomotic strategies. It is the authors’ practice to use the coloplasty for those situations in which a side-to-end anastomosis or colon J-pouch is not feasible. This technique may be of some value in those patients who require some form of reconstruction but need the length provided with an end-to-end anastomosis to reach the top of the distal bowel. Further data are needed before this reconstruction procedure can be recommended as preferable to the simple end-to-end anastomosis.
Anastomotic Method
Hand-sewn anastomosis
Any of the earlier reconstruction procedures can be hand sewn in 1 or 2 layers with either interrupted or continuous sutures. A hand-sewn colorectal anastomosis is generally constructed from the abdominal incision. Sutures are placed evenly at approximately 4 to 5 mm from the previous suture and without excessive tension to avoid tissue ischemia. For more difficult lower pelvic anastomoses, it is easiest to place the sutures on the distal side first and then parachute the proximal bowel down to the distal end. The mucosa can be inverted by using Lembert sutures or by laying the knots inside the lumen.
For anastomoses at the top of the anus, with or without mucosectomy, a hand-sewn coloanal anastomosis may be performed transanally. This procedure is generally performed in lithotomy with the aid of an abdominal operator. A retractor such as the Parks instrument is necessary for proper visualization. Then the colon is passed through the anal cuff and full-thickness bites of an absorbable suture are placed every 5 mm. The authors elect to divert these patients with a proximal loop ileostomy for at least 2 months.
Double purse-string stapled anastomosis
The double purse-string stapled anastomosis technique is performed with the distal bowel open. Surgeons sometimes force the stapler to the top of the rectum thereby producing rectal lacerations or, alternatively, perform an anastomosis to the anterior midrectum because the stapler is not easily passable to the top of the acute Hartmann. Thus, the advantages for this technique include the capability of the surgeon to manipulate the circular stapler in a cephalad direction to the top of a particularly curvy rectum. First, a continuous monofilament suture is purse-stringed along the open rectal or anal cuff and a second purse-string suture is placed in the colon. The circular stapler is placed through the anus and can be advanced to the top of the rectum by introducing a digit through the open rectal cuff and gently guiding the stapler to the top of the cuff. The anastomotic pouch or neorectum can then be stapled as per routine and the anastomosis air tested for leaks as described now for the double-stapled technique.
Double-stapled anastomosis
The double-stapled technique has the advantage of reducing potential fecal contamination by stapling the rectal cuff. First, a transverse stapler is placed below the cancer to divide the rectum from the specimen. Then a monofilament purse-string suture is placed around the cut edge of the distal lower colon. A circular stapler anvil is then inserted into the descending colon while the purse string is tied. The authors elect to air-leak test the stapled acute Hartmann or distal rectal stump to reduce the possibility of unidentified air leak from the transverse staple line corners. If an air leak is identified from the acute Hartmann, then the circular stapler obturator is brought out through the rent in the staple line. If there is no air leak, the circular stapler is advanced through the rectum to the top of the rectal cuff and the obturator is opened in the center of the transverse staple line. The mobilized descending colon and anvil is then mated to the top of the circular stapler, closed, and fired, resulting in the finished anastomosis. The anastomosis is then air tested with a proctoscope while the anastomosis is under irrigant.
Role of Temporary Fecal Diversion
The incidence of anastomotic complications following low pelvic anastomosis is highest as the anastomosis draws closer to the anus. In fact, the risk of anastomotic complications is reported to range from 4.9% to 17% in the literature. In addition, a history of radiation therapy, immunosuppression, and diabetes increases the risk of anastomotic complications. Given that the sequelae of a leaking anastomosis remain a major source of morbidity and mortality after reconstruction for proctectomy, some surgeons have taken to routinely diverting patients following a low anastomosis. Others have recommended temporary fecal diversion in the setting of low anastomosis and after preoperative irradiation, to reduce the consequences of a leak. Initially, proximal fecal diversion was believed to diminish the morbidity resulting from leakage and reduce the likelihood of an emergency reoperation. However, recently there have been 2 meta-analyses that have demonstrated a reduced clinical anastomotic leak rate in patients with defunctioning stoma as compared with no fecal diversion. Fecal diversion is considered for patients with a low colorectal anastomosis if (1) they have undergone preoperative radiation therapy, (2) have a pelvic pouch or coloplasty reconstruction, or (3) technical issues are experienced during reconstruction.
APR
The APR procedure involves en bloc resection of the rectosigmoid, the rectum, and the anus along with the surrounding mesentery, mesorectum, and perianal soft tissues. In the situation in which the operating surgeon is unable to obtain a 1-cm distal margin, the slimmest acceptable margin, an APR is considered. In addition to this scenario, preoperative factors that point toward a benefit to APR include patient preference, poor preoperative continence, and/or tumors that obviously grow into the anal sphincter mechanism. It is clear that a patient with preexisting fecal incontinence or sphincter injury has better function following an APR or other procedure with permanent colostomy than by the heroic attempt to save the anal sphincter and reestablish intestinal continuity. The technical details of APR are beyond the scope of this article, but it should be understood that one should avoid the waist observed in APR specimens, that is, a narrowing of the specimen that occurs as the dissection proceeds from superiorly into the levator hiatus. Unlike the original description of APRs in which the levator was transected off the lateral pelvic side wall, present techniques have evolved toward a closer dissection to the rectum at the proximal anal canal. This represents a potentially compromised radial margin at that point and probably explains the observed higher incidence of local failure after APR.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree