CASE HISTORY
A 39-year-old woman presented to her physician with a 6-month history of polyuria, nocturia, 5 lb (2.3 kg) weight loss and vaginal itch. She was overweight at 168 lb (76 kg) for her height of 5 feet 4 inches (163 cm) (body mass index [BMI] 29) and smoked 10 cigarettes per day. Blood pressure was 138/88 mmHg. A random blood glucose was raised at 14.4 mmol/L (260 mg/dL) with a repeat fasting glucose of 10 mmol/L (180 mg/dL) the following day, confirming the diagnosis of diabetes. A spot early morning urine test for microalbumin was negative. Fasting lipids showed a total cholesterol of 5.7 mmol/L (220 mg/dL), triglycerides 3.3 mmol/L (294 mg/dL), lowdensity lipoprotein (LDL) cholesterol 2.9 mmo/L (111 mg/dL), and high-density lipoprotein (HDL) cholesterol 0.9mmo/L (36 mg/dL). Ophthalmologic evaluation revealed early signs of non-proliferative diabetic retinopathy in the left eye.
She had four previous children. Her last pregnancy, 8 years prior to the current pregnancy, had been complicated by GDM, diagnosed on screening at 28 weeks, for which she was treated with diet initially and subsequently insulin from 34 weeks of gestation. Her weight gain during that pregnancy was 45 lbs (20.4 kg). She delivered a 4400-g healthy infant by cesarean section at 38 weeks of gestation. Her insulin was discontinued at delivery. Six weeks postpartum, she was informed that her fasting plasma glucose was normal and she was advised to have the test repeated in 1 year. She breastfed for 4 months and managed to loose 30 lb (13.6 kg) in the first 6 months postpartum through lifestyle changes. At her request a tubal ligation was performed 9 months after delivery. Contrary to advice she was subsequently lost to review until she represented with classical hyperglycemic symptoms.
Once the diagnosis of diabetes mellitus was confirmed, she expressed concern about her 21-year-old daughter who was diagnosed with GDM at week 28 of her first and only pregnancy. She asked if her daughter was also at risk of developing overt diabetes in the future, and if so, were there any possibilities for prevention?
- Why was the patient asked to return one year after delivery, in spite of a normal 6 weeks postpartum?
- What would you advise the patient’s daughter following the birth of her own child in order to prevent the development of diabetes mellitus 2?
BACKGROUND
It was recognized more than 100 years ago that hyperglycemia in pregnant women may disappear after delivery but return years later.1 In the late 1940s and early 1950s, several retrospective studies demonstrated a high perinatal morbidity and mortality in infants of mothers who developed diabetes years later. These findings stimulated the investigation of carbohydrate metabolism in pregnancy with the use of oral and intravenous glucose tolerance tests, in order to detect hyperglycemia with the aimof improving maternal/fetal outcome.
The development of hyperglycemia years after the diagnosis of what is known today as GDM was first recognized by Duncan in 1892.2 He made three remarkable statements in the conclusion of his pioneer article on “Diabetes and pregnancy”:
- Diabetes may come on during pregnancy
- Diabetes may occur only during pregnancy and be absent at other times
- Diabetes may cease with the termination of pregnancy, recurring some time after delivery.
In the decades between 1930 and 1950, it was shown that at the diagnosis of diabetes mellitus, women frequently gave an obstetric history of macrosomic infants, unexplained intrauterine deaths, and neonatal morbidity and mortality.3–5 A perinatal death rate of 15.4% was reported in the 5 years preceding the diagnosis of diabetes compared with a perinatal mortality rate of 6% in women delivering 5–12 years before the diagnosis of diabetes.4
The first study of the use of an oral glucose tolerance test (OGTT) in pregnancy was by Wilkerson and Remein in Boston.6 The aim of the study was “to evaluate the effect of insulin treatment on the outcome of pregnancy in women with abnormal carbohydrate tolerance to determine if such treatment would:
- Result in a lower rate of fetal wastage and other complications of pregnancy
- Delay the onset of diabetes in pregnant women in the prediabetic stage
- Decrease the chance of diabetes occurring in the live births”.
O’Sullivan and Mahan,7 using the same material collected by Wilkerson and Remein, established the criteria for the diagnosis of GDM, based not on perinatal outcome, but on the cumulative incidence of future diabetes in women with GDM; they reported an incidence of 67% of diabetes at 5 ½ years following delivery. In 1960 Jackson8 from South Africa suggested that a “temporary” diabetic state or significant impairment of glucose tolerance during pregnancy indicated a state of potentially permanent diabetes in the mother.
Since this first observation of overt diabetes developing years after the diagnosis of GDM, several clinical studies have confirmed these findings. Mestman et al9 followed 360 mostly Latina GDM women for up to 5 years after delivery. Of 51 women with an elevated fasting blood glucose of greater than 5.5 mmol/L (100 mg/dL) during pregnancy, only four reverted to a normal OGTT 6 weeks postpartum. Of 181 women with an abnormal OGTT but normal fasting blood glucose in pregnancy, 23 (12.7%) developed overt diabetes (fasting blood glucose > 5.6 mmol/L [100 mg/dL]) and 59 (32.6%) of them developed impaired glucose tolerance (IGT). Other investigators confirmed the above findings.10–12
Based on these findings, the routine use of an OGTT 4–8 weeks postpartum was recommended. Currently, those women with positive tests are referred to healthcare professionals for long-term follow-up, including diabetes education, lifestyle modification, screening, and management of cardiovascular risk factors in order to prevent or delay the deterioration of carbohydrate intolerance and reduce the risk of future cardiovascular disease. This approach is supported by a number of studies in the last two decades which have shown the effectiveness of aggressive lifestyle modification, physical activity, and pharmacologic therapies in the prevention of Type 2 diabetes. It is the responsibility of healthcare professionals caring for women with GDM to encourage them to have an OGTT in the first few weeks after delivery and to make provision for their proper education and long-term care.
RISK FACTORS FOR THE DEVELOPMENT OF TYPE 2 DIABETES IN WOMEN WITH GESTATIONAL DIABETES MELLITUS
Kim et al13 presented a systematic review of 28 articles published between 1965 and 2001 on GDM and risk of future Type 2 diabetes. All women were examined 6 weeks to 28 years postpartum, and the cumulative incidence of diabetes ranged from 2.6% to 70%. The authors made several interesting observations.
- Once diagnosed with GDM, women from mixed or non-white cohorts seemed to progress to Type 2 diabetes at similar rates.
- The progression to Type 2 diabetes increased steeply within the first 5 years after delivery and then appeared to plateau.
- Women of white ethnicity converted at a similar rate to the other ethnic groups, but it was difficult to assess this because of the relatively few studies in this population.
- Elevated pregnancy fasting glucose before 24–26 weeks of gestation predicted Type 2 diabetes, except when more specific measures of pancreatic beta cell function were concomitantly examined, such as insulin secretion.
- Other risk factors had inconsistent or little predictive value after adjustment for other variables, such as BMI, maternal age, previous history of GDM, family history of diabetes, and parity. In many studies reviewed by the authors, some of the above risk factors were associated in univariate analyses, but not in multivariate analysis. Kjos et al14 studied 671 Latino women with GDM, all
of whom had a normal OGTT 4–16 weeks postpartum. The subjects underwent at least one OGTT within the following 7.5 years. Life table analysis revealed a 47% cumulative incidence rate of Type 2 diabetes 5 years after delivery. They identified four variables as independent predictors for the development of Type 2 diabetes:
- Glucose area under the postpartum OGTT (4–16 weeks) curve (933 ± 189mmol/L/min)
- Gestational age at the time of the diagnosis of GDM (28.1 ± 0.3 weeks)
- Glucose area under the pregnancy OGTT (1744 ± 277mmol/L/min)
- Highest fasting serum glucose concentration (6.2 ± 1.5mmol/L) (111.7 ± 27.0mg/dl) during pregnancy.
The area under the postpartum OGTT, however, provided the best discrimination between high-risk and low-risk individuals.
Peters et al15 in a group of Latino women with GDM reported that an additional pregnancy increased the risk of developing Ty pe 2 diabetes (RR 3.34, 95% Cl 1.80–13.8) as well as weight gain within 7.5 years after delivery (RR 1.95, 95% Cl 1.63–2.33).
Not only are women with GDM at higher risk of developing overt diabetes but those with a slight elevation in blood glucose during a screening oral glucose challenge test (OGCT) or a diagnostic OGTT are at similar risks.16–18 In a retrospective study17 women with an abnormal OGCT and one abnormal glucose value during the OGTT were followed for a median period of 8.8 years. Type 2 diabetes was ascertained by ICD-9 codes or pharmacy or laboratory data. The higher the elevation in glucose values during pregnancy, the greater the risk for subsequent development of Type 2 diabetes. The above findings are supported by studies showing a decrease in beta cell function in women with one abnormal value in the OGTT compared to women with a negative OGTT.18 Furthermore, it was reported that isolated hyperglycemia at 1 hour during an OGTT is associated with postpartum hyperglycemia, insulin resistance, and beta cell dysfunction.19
Table 24.1 Predictors of gestational diabetes mellitus (GDM) and Type 2 diabetes.
| GDM | Type 2 diabetes |
| Obesity | Obesity |
| Family history of Type 2 diabetes | Family history of Type 2 diabetes |
| Waist-to-hip ratio | Waist-to-hip ratio |
| Previous history of GDM | Previous history of GDM |
| Ethnic background | Ethnic background |
| Hypertension | Hypertension |
| Previous history of macrosomia | Dyslipidemia |
| Advanced maternal age Cigarette smoking | Low birthweight Physical inactivity Polycystic ovarian syndrome Cigarette smoking |
As shown in Table 24.1 risk factors for developing GDM are very similar to those for Type 2 diabetes.
LONG-TERM CARDIOVASCULAR COMPLICATIONS IN WOMEN WITH GESTATIONAL DIABETES MELLITUS
Women with diabetes mellitus are at increased risk of developing cardiovascular disease with amortality higher than in men.20 A possible explanation for this observation is that women are less likely to receive the same aggressive treatment for lipids, hypertension, and hemoglobin A1c (HbA1c) than men.21,22 In one of the earliest studies,23 89 women with GDM were interviewed 12–18 years after their pregnancies; 58 (65.2%) of them had developed overt diabetes. The incidence of hypertension was 44.8% in the GDM group versus 12.9% in the control group. Five women had had a stroke, four a myocardial infarction, and two were on chronic dialysis. In a preliminary analysis of the Boston Gestational Diabetes Study, O’Sullivan24 showed that during 26 years of follow-up, postpartum rates of mortality, hypertension, and dyslipidemia were significantly higher in the GDM group compared with controls. He also reported more electrocardiogram (ECG) abnormalities in the GDM women with three- to five-fold more cases of myocardial infarction and angina.
Carr et al25 studied 994 parous women with Type 2 diabetes, who had a first-degree relative with Type 2 diabetes, 29.9 years after the index pregnancy (range 1.2–74.0). Three hundred and thirty-two women (33.4%) gave a history of GDM, and 662 (67.6%) had no history of GDM. BMI, race, and ethnicity were similar between the two groups with each composed of about one-third Caucasians, African Americans, and Latinas. The mean age at the time of the study was 52.4 years in women without a previous history of GDM and 48.6 years in those with a history of GDM (p < 0.001). Women with previous GDM were more likely to have developed Type 2 diabetes, to have been diagnosed with the metabolic syndrome, and to have suffered a cardiovascular disease (CVD) event compared with women without prior GDM.
Shah et al26 identified 8191 women diagnosed with GDM in Ontario, Canada between 1994 and 1997, and 81 262 women who had a live birth without GDM, with a median follow-up of 11.5 years. Diabetes, mostly Type 2, developed during follow-up in 27% of the women with GDM and 3.2% of the women without GDM. The authors concluded that women with GDM had a substantially increased risk for CVD events (HR 1.71; 95% CI 1.08–2.69) in later life compared with women without GDM. Much of this increased risk was attributable to the subsequent development of Type 2 diabetes.
RELATIONSHIP BETWEEN GESTATIONAL DIABETES MELLITUS AND METABOLIC SYNDROME
The similarities in risk factors for the development of GDM and Type 2 diabetes have been recognized for the last three decades (Table 24.1). In addition to insulin resistance, compensatory hyperinsulinemia and beta cell dysfunction, abnormalities in lipid metabolism, hypertension, and obesity are present in both clinical entities. It has been suggested that both conditions are the same disorder.27,28 Women with a history of previous GDM, studied at the time of normal glucose tolerance, present the hallmarks of insulin resistance and hyperinsulinemia.29
The metabolic syndrome or insulin resistance syndrome is a predictor for development of cardiovascular complications,30 with a prevalence of 20–40% in an American adult population.31 The diagnostic criteria suggested by the National Cholesterol Education Program (NCEP) expert panel32 require the presence of three of five of the following for diagnosis: (1) blood pressure 130/85 mmHg or greater; (2) HDL cholesterol less than 1.03 mmol/L (40 mg/dL) in men and 1.29 mmol/L (50 mg/dL) in women; (3) serum triglycerides greater than 1.69 mmol/L (150 mg/dL); (4) abdominal obesity defined as a waist circumference greater than 40 inches (100 cm) in men and 36 inches (92 cm) in women; and (5) a fasting serum glucose of greater than 5.5 mmol/L (100 mg/dL). Hyperinsulinemia is a constant feature. Other manifestations of the metabolic syndrome are:
- Hyperuricemia and gout
- Acanthosis nigricans
- Sleep apnea
- Polycystic ovary syndrome
- Fatty liver disease with steatosis, fibrosis, and cirrhosis.
The International Diabetes Federation (IDF) proposed in 200433 the following criteria:
- Increased waist circumference
- Serum triglycerides greater than 1.7 mmol/L (150 mg/ dL) or treatment for elevated triglycerides
- HDL cholesterol less than 1.03 mmol/L (40 mg/dL) in men and less than 1.29 mmol/L (50 mg/dL) in women, or treatment for low HDL cholesterol
- Systolic blood pressure greater than 130 mmHg, diastolic blood pressure greater than 85 mmHg, or treatment for hypertension
- Fasting plasma glucose greater than 5.5mmol/L (100 mg/dL), or previously diagnosed Type 2 diabetes.
In a study in the US, the IDF criteria categorized 15–20% more adults with the metabolic syndrome than the NCEP criteria.34
Type 2 diabetes develops in subjects with defects in insulin secretion in the presence of insulin resistance. Women with a history of GDM have been studied at different times following the index pregnancy to determine insulin action and insulin resistance in the presence of a normal glucose tolerance test.29,35 In spite of having a normal glucose tolerance, these women were found to have defects in both insulin secretion and insulin action.
Kjos et al36 studied lipid metabolism in a group of Latina women with GDM followed for 36 months. At 6–12 weeks postpartum, serum triglycerides were higher and HDL cholesterol lower in those women who later developed diabetes mellitus as compared with women who did not develop diabetes. This is consistent with findings in prediabetic patients.37
Birthweight is inversely related to subsequent risk for Type 2 diabetes, insulin resistance, and other features of the metabolic syndrome.38 Both low birthweig ht (<2500g) and high birthweight (> 4500 g) are associated with the future development of GDM and Type 2 diabetes (the latter phenomenon is most likely explained by the presence of obesity and hyperglycemia during pregnancy).39
Clark et al40 hypothesized that GDM could manifest many of the characteristics of the metabolic syndrome. They compared the metabolic profile in 179 women (91 African American, 81 Caucasian, and seven of other ethnic groups), 52 of whom had a history of GDM while 127 did not, as determined by a normal 1-hour glucose screen. Women with GDM had a higher prepregnancy BMI, c-peptide, insulin levels fasting and at 2 hours post-prandially, and fasting free fatty acid, and a lower HDL cholesterol during pregnancy (16–33 weeks). The authors suggested that GDM might be viewed as a component of the metabolic syndrome. It also provides an excellent model for study and prevention of Type 2 diabetes in a relatively young age group.
Launeberg et al41 studied a population of women, mean age 43 years, with prior diet-treated GDM, 75% of whom were of Danish origin, with the aim of estimating the prevalence of the metabolic syndrome 9.8 years (range 6.4–17.2 years) after the index pregnancy; the control group comprised 1000 women (95% Danish). The main outcome measures were BMI, glucose tolerance, blood pressure, lipids, and insulin resistance, measured by fasting insulin levels. The prevalence of the metabolic syndrome was 40% in women with prior GDM, three times higher than in the control group.
The above studies strongly support the hypothesis that GDM has many features in common with the metabolic syndrome. These observations have very important implications not only for the detection of patients at risk at a young age, but also as they offer the potential to prevent diabetes in the offspring of women with GDM. As stated by Norbert Freinkel42 in the opening address of the First International workshop on GDM, “… with GDM women, we may be able to unmask a population at greater risk for permanent diabetes than under non-gravid conditions and use it to evaluate the efficacy of preventive measures”.
PREVENTION OF TYPE 2 DIABETES
Since it is known that most women with a history of GDM are at higher risk for the development of Type 2 diabetes, simple and practical measures in early detection of prediabetes and prevention of Type 2 diabetes should have a major impact on decreasing cardiovascular morbidity and mortality. One of the barriers is the lack of perception and understanding after delivery on the part of the women (and perhaps also that of the healthcare providers) of the seriousness and consequences of diabetes mellitus. Among a selected group of 217 mostly white, affluent, and well-educated women with a previous history of GDM, 7% believed that they had almost no chance of developing diabetes, 35% a slight chance, 41% a moderate chance, and 16% a high chance; only 31% reported engaging in lifestyle modification.43 Therefore, patient education in lifestyle modification and encouragement to return for glucose testing at regular intervals are important tools for the healthcare professional in the subsequent follow-up of women with GDM.
Physical inactivity and obesity are well-known risk factors for the development of Type 2 diabetes. It was postulated that lifestyle modification, including weight loss, decreasing the total amount of ingested calories, increasing the amount of fiber in the diet, and increasing daily physical activity could delay or prevent the development of Type 2 diabetes in those subjects at higher risk.44,45 In the study by Helmrich et al45 among a group of women, the incidence of Type 2 diabetes was reduced by a third through vigorous exercise independent of a family history of diabetes.
In the Diabetes Prevention Program,46 3234 subjects of both genders aged over 25 years with a BMI of 24 or higher and a fasting serum glucose concentration of 5.3–6.9mmol/L (95–125mg/dL]) or a 2-hour value of 7.8–11.0 mmol/L (140–199 mg/dL) after a 75-g g lucose load were eligible for the study. The subjects were assigned to four different groups:
- Intensive lifestyle changes under strict supervision with the aim of reducing weight by 7% with diet and 150 minutes of weekly exercise
- Metformin 850mg twice a day, combined with a meal plan and physical activity advice
- Troglitazone (but this arm was discontinued due to drug toxicity)
- A control group.
The incidence of diabetes was reduced by 58% with lifestyle intervention and by 31% with metformin compared with placebo. The average follow-up of the study was 2.8 years. In the intensive lifestyle group, 50% of the participants had achieved the goal of weight loss of at least 7% or more at the end of 24 weeks; 74% of the participants met the goal of at least 150 minutes of weekly exercise by 24 weeks. The adherence to metformin was 72%. The average weight loss was 0.1, 2.1, and 5.6 kg in the placebo, metformin, and lifestyle intervention groups, respectively (p < 0.001). These effects were similar in men and women and in all racial and ethnic groups. Metformin was more effective in younger individuals.
In Finland,47 552 middle-age overweight subjects with impaired glucose tolerance were randomly assigned to either an intervention or a control group. In the former, individual counseling aimed at reducing weight by decreasing intake of dietary fat, increasing intake of dietary fiber, and increasing physical activity was given. The cumulative incidence of diabetes after 4 years was 11% (95% CI 6–15%) in the intervention group and 23% (95% CI 17–29%) in the control group. During the trial, the risk of diabetes was reduced by 58% in the intervention group and was directly associated with changes in lifestyle.
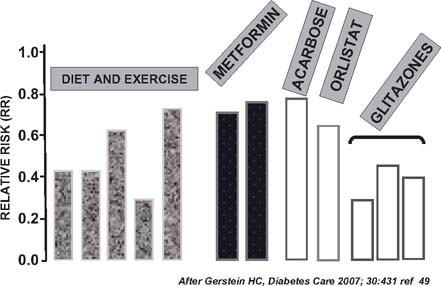
Drug therapy is also an important tool in preventing or delaying the onset of Type 2 diabetes. The TRIPOD study48 involved Latino women with previous GDM and no diabetes at entry. They were followed for 30 months and compared with a placebo group matched for age, BMI, and parity. The treated group of 133 women received troglitazone 400 mg a day. However, the study was terminated after 30 months because of withdrawal of the drug from the market due to hepatotoxicity. The incidence of diabetes was 12.1% in the control group and 5.4% in the treated group (p < 0.01). The protection from diabetes persisted for 8 months after the drug was stopped and it was associated with preservation of pancreatic beta cell function assessed by an intravenous glucose tolerance test. This beneficial effect appeared to be mediated by a reduction in the secretory demands placed on the beta cell by chronic insulin resistance. Other studies, summarized by Gerstein,49 showed the beneficial effect of other drugs in reducing the incidence of Type 2 diabetes in persons with prediabetes (Fig. 24.1).
The natural history of Type 2 diabetes is shown in Fig. 24.2. Individuals with insulin resistance maintain normal glycemia because they produce more insulin. Even with normal glucose levels, a significant proportion of them suffer from asymptomatic hypertension, dyslipidemia, and central obesity. If insulin resistance is not corrected, the pancreas is unable to maintain normoglycemia and postprandial hyperglycemia develops (the prediabetic state). Many of these women will develop GDM while others are alternatively diagnosed on admission to hospital after significant stress, such as a myocardial infarct or stroke. With time, the production of insulin further decreases and fasting hyperglycemia develops. At the time of diagnosis of Type 2 diabetes macro-and micro-vascular complications are present in 10–30% of individuals.50,51
Fig. 24.1 Pharmacologic and non-pharmacologic measures to delay or prevent the onset of Type 2 diabetes. (After Gerstein49.)