Highlights
- •
Lung-only metastases in mCSPC patients showed better outcomes than other visceral metastases.
- •
Bone metastasis volume significantly influenced CRPC-free survival, unlike lung metastases.
- •
Prognoses for patients with lung metastases and low- or high-volume bone metastases were similar.
- •
Lung metastases may not be a high-volume risk factor, highlighting the need for tailored treatments.
Abstract
Background
Androgen receptor-signaling inhibitors (ARSIs) have significantly changed the preferred treatments for metastatic castration-sensitive prostate cancer (mCSPC). Despite such advances, the prognostic significance of metastases at specific sites remains unclear. This study evaluated how metastatic site affected the oncological outcomes of mCSPC patients.
Methods
This retrospective multicenter study included 716 mCSPC patients receiving androgen- deprivation therapy (ADT) alone, combined androgen blockade (CAB) therapy, or both ARSI and ADT (ARSI doublet) from February 2018 to June 2023. All patients were categorized based on their metastatic sites. The primary endpoint was the time to castration-resistant prostate cancer (CRPC) development; the secondary endpoints were progression-free survival 2 (PFS2), cancer-specific survival (CSS), and overall survival (OS). Kaplan-Meier curves and multivariate Cox’s regression models were used to analyze the survival outcomes. We stratified mCSPC patients with bone metastases by the volumes of such metastases and lung metastasis status, and explored the clinical significance of lung metastasis.
Results
Patients with lung-only metastases experienced better outcomes than those with other visceral metastases. On multivariate analysis, the bone metastasis volume, but not lung metastasis status, significantly affected CRPC-free survival status. No significant difference in any of CRPC, PFS2, CSS, or OS status was apparent among patients with bone metastases with or without lung metastases. In terms of interaction, lung metastasis did not significantly affect the prognoses of patients with either low- or high-volume bone metastases.
Conclusion
In the present era of ARSI doublet therapy, lung-only metastases in mCSPC patients were associated with favorable outcomes. The negative prognostic effects of lung metastases were much lower than was the bone metastasis volume, indicating that treatments targeting low-volume disease may be adequate even when lung metastases are apparent.
1
Introduction
Androgen receptor signaling inhibitors (ARSIs) have transformed the management of metastatic castration-sensitive prostate cancer (mCSPC) [ ]. Over the past 2 decades, treatment has evolved from androgen deprivation therapy (ADT) either alone or combined with androgen blockade (CAB), to addition of ARSIs, chemotherapy, or combinations thereof (depending on the tumor characteristics) [ , ]. Although triplet ARSI, docetaxel, and ADT therapies are efficacious, especially in patients with high-volume mCSPC, most current clinical guidelines suggest that an both ARSI and ADT (ARSI doublet) should be used to treat mCSPC [ , ].
Despite the advances in treatment, the prognostic significance of mCSPC metastases at specific sites remains unclear. Previous studies have explored the effects of the metastatic location on prognosis. Some authors used small institutional datasets [ , ]; others analyzed large-scale populations [ , ]; and several leveraged pooled data from randomized clinical trials [ ]. However, many of the studies predated the widespread use of ARSIs, emphasizing the need for further work in the current era in which upfront ARSI doublet therapy is widely employed to treat mCSPC.
In this study, we analyzed real-world data collected after abiraterone (AAP) was approved to treat high-risk mCSPC in Japan and upfront ARSI doublet therapy became covered by the health insurance system. First, we assessed the clinical significance of metastatic sites in this context. Notably, a lung metastasis is considered to be a visceral metastasis; patients with lung involvement are considered ‘high-volume’ by the CHAARTED criteria [ ]. However, a previous report suggested that lung metastasis was associated with a more favorable mCSPC prognosis than other metastases [ ]. Several studies found that, of the visceral metastases, liver metastases were associated with poorer prognosis than only lung metastases [ ]. Therefore, we explored whether lung metastases affected the oncological outcomes of mCSPC patients with bone metastases.
2
Patients and methods
2.1
Patients
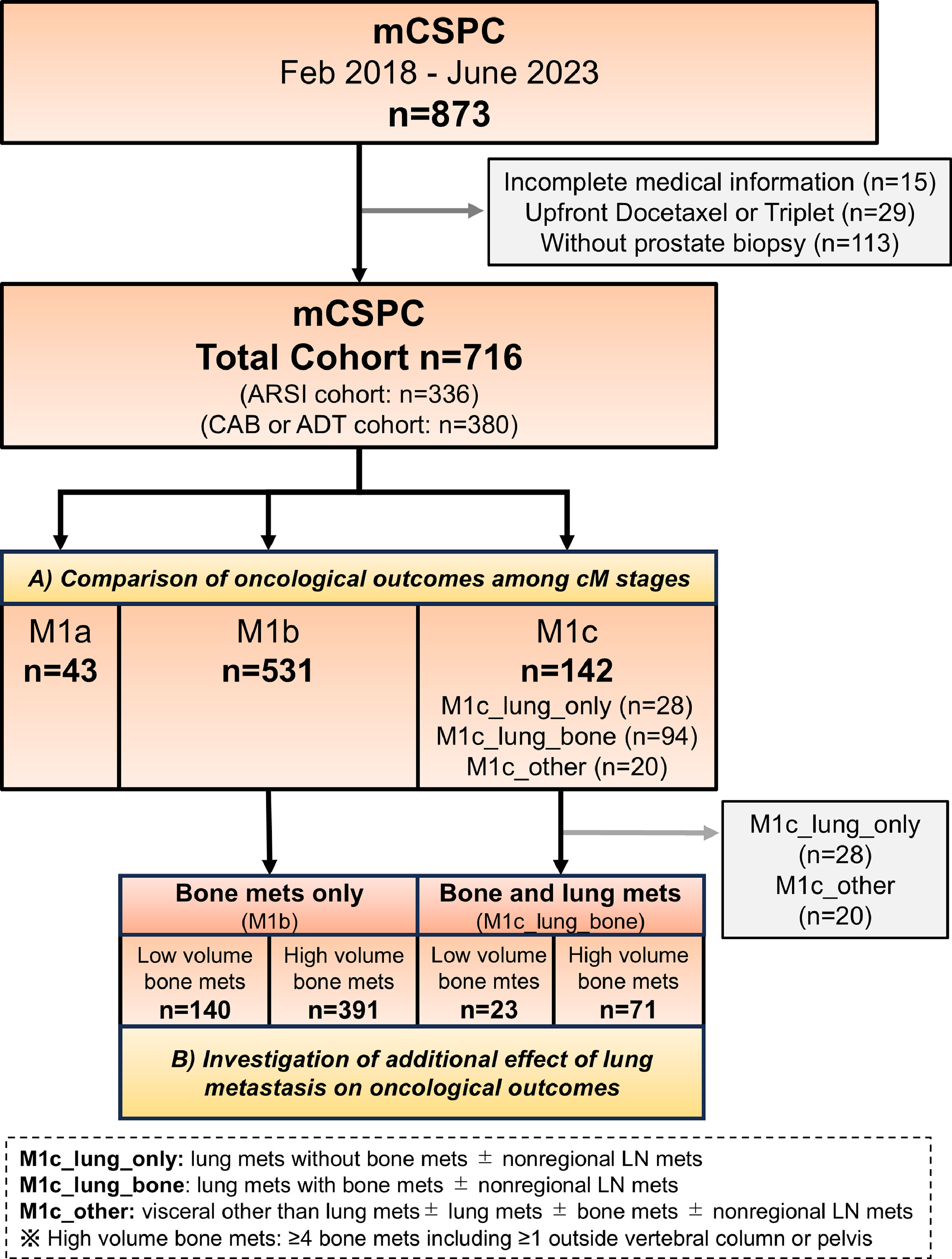
This retrospective multicenter study enrolled consecutive patients with mCSPC treated with ADT alone, CAB, or ARSIs with ADT at 4 university hospitals and 15 affiliated general hospitals from February 2018 to June 2023 (JIKEI-YAYOI Collaborative Group). ADT included orchiectomy and luteinizing hormone-releasing hormone agonists and antagonists. CAB was defined as surgical or medical ADT combined with bicalutamide. ARSI patients received enzalutamide, apalutamide, or AAP in combination with ADT. After first-line therapies, sequential treatments were administered at the discretion of physicians. We excluded patients whose clinical details were incomplete (n = 15), those who received upfront docetaxel or triplet therapy (n = 29), and patients who did not undergo prostate biopsy (n = 113). The study protocol was approved by the Institutional Review Board of Jikei University (approval number 33–260[10878]).
2.2
Clinicopathological data and study outcomes
Patient demographics, clinicopathological characteristics, treatment strategies, and follow-up results were collected. Abdominal and pelvic computed tomography and bone scans were used to stage prostate cancer. The primary efficacy endpoint was the time to development of castration-resistant prostate cancer (CRPC). The secondary endpoints were progression-free survival 2 (PFS2), cancer-specific survival (CSS), and overall survival (OS). These outcomes are defined in the Supplementary Information. The prostate-specific antigen (PSA) concentration and serum hematological and biochemical marker levels were recorded, and liver function tests performed at baseline, monthly during the first year, and at least every 2 months thereafter. Timing commenced on the date of ADT initiation. The extent of disease (EOD) score was categorized into 5 distinct levels based on the degree of bone metastasis observed on imaging. The grading system is as follows: 0 indicates a normal scan; 1 represents fewer than 6 bone metastases, each measuring ≤50% of the size of a vertebral body (a single lesion equivalent in size to a vertebral body is considered as 2 lesions); 2 comprises 6 to 20 bone metastases; 3 includes more than 20 metastases but does not meet the criteria for a “superscan”; and 4 is defined as either a “superscan” or bone metastases affecting >75% of the ribs, vertebrae, and pelvic bones. Radiation therapy to the primary tumor was administered at the physician’s discretion.
2.3
Statistical analysis
Continuous parameters are presented as medians with interquartile ranges (IQRs). Chi-squared, Kruskal–Wallis, and Mann–Whitney U-tests were used as appropriate to evaluate associations between histological types and clinicopathological variables.
Two separate analyses were conducted. First, we evaluated the prognostic significances of various metastatic sites. Patients were categorized into the following groups: M1a (nonregional lymph node metastases only); M1b (bone metastases only; no visceral metastases); M1c_lung_only (lung metastases with no bone metastases and no other visceral metastases, such as liver, with or without nonregional lymph node metastases); M1c_lung_bone (lung metastases with bone metastases, with or without nonregional lymph node metastases); and M1c_other (nonpulmonary visceral metastases, with or without lung, bone, or nonregional lymph node metastases). Next, we focused on patients with bone metastases; they were stratified by volume of bone metastases and lung metastasis status. Comparisons of these groups revealed the clinical significance of lung metastases in patients with bone metastases. In accordance with the CHAARTED criteria [ ], high-volume bone metastases are defined as the presence of 4 or more bone metastases, including at least 1 metastasis outside of the vertebral column or pelvis.
All survivals were estimated using the Kaplan–Meier method. Differences between treatment strategies were assessed using the log-rank test. Further comparisons employed multivariate Cox’s regression models, which yielded hazard ratios (HRs) with 95% confidence intervals (95% CIs). Interactions among subgroups were tested by adding multiplicative terms to the relevant Cox’s model. A P -value < 0.05 was considered statistically significant. All statistical analyses were performed using Stata software version 13.1 (StataCorp LP, College Station, TX, USA) and R software (The R Foundation for Statistical Computing, Vienna, Austria).
3
Results
3.1
Patient demographics
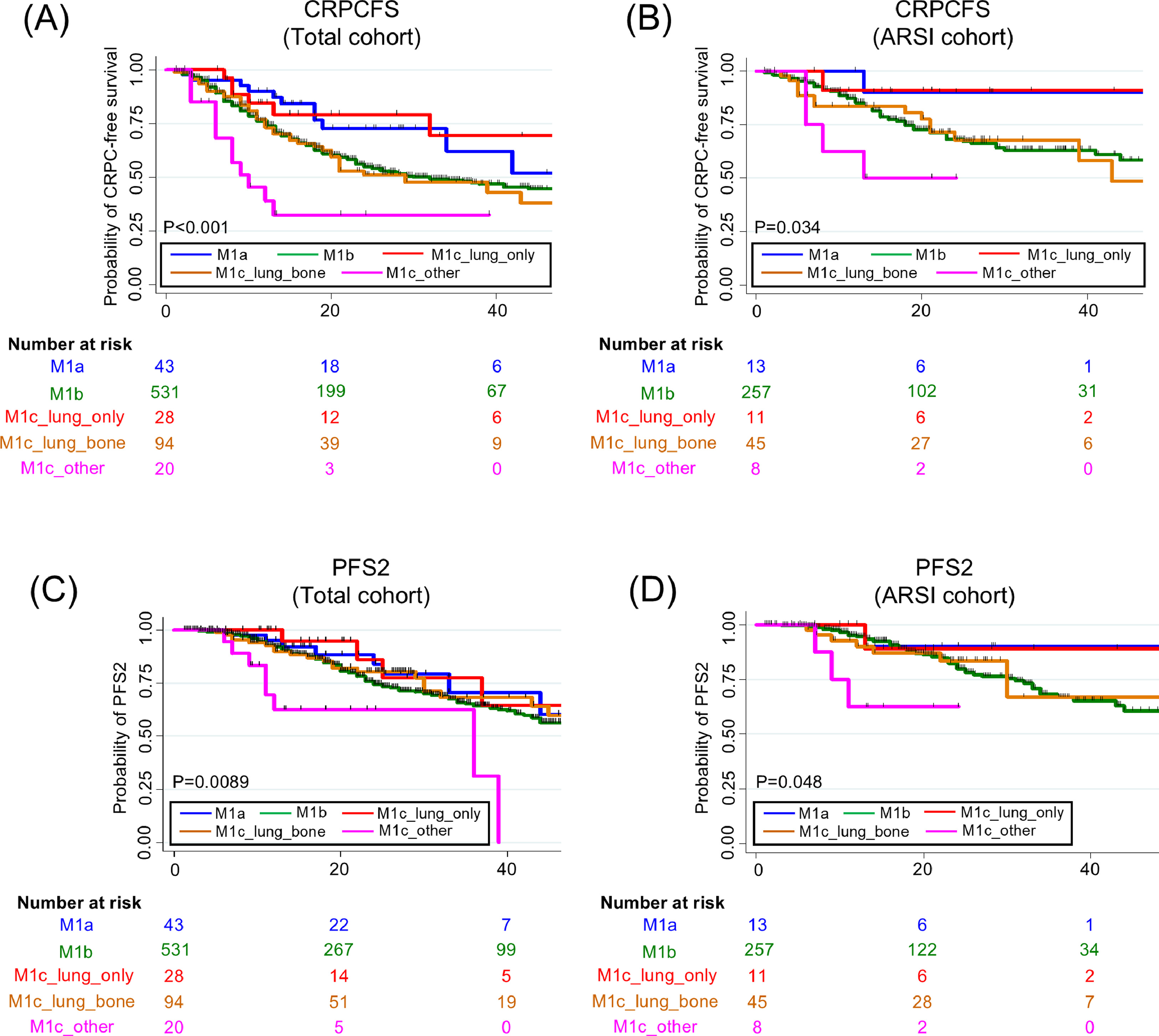
The study population included 716 patients as follows: 43 (6.0%) in the M1a group, 531 (74.2%) in the M1b group, 28 (3.9%) in the M1c_lung_only group, 94 (13.1%) in the M1c_lung_bone group, and 20 (2.8%) in the M1c_other group ( Fig. 1 and Table 1 ). The median age was 75 years (IQR: 69–80 years) and the median follow-up was 23 months (IQR: 12–37 months). Significant among-group differences were observed in terms of the baseline PSA level, EOD score, and radiation to primary site ( P < 0.05; Table 1 ). Of the 20 cases in the M1c_other group, 17 had liver metastases. Other visceral metastases included adrenal metastases in 3 cases and brain metastases in 1. Of the total cohort, 336 (46.9%) received ARSI doublet therapy. Supplementary Table 1 summarizes the baseline characteristics of patients in the ARSI cohort (thus mCSPC patients who received ARSI doublet therapy).
| Covariant | Total | M1a | M1b | M1c_lung_only | M1c_lung_bone | M1c_other | P -value |
|---|---|---|---|---|---|---|---|
| No of Patients | 716 | 43 | 531 | 28 | 94 | 20 | |
| Follow-up duration (mo) median (IQR) | 23 (12–37) | 24 (14–37) | 23 (12–38) | 19 (11–33) | 24 (11–37) | 16 (9–23) | 0.061 |
| Age (y), median (IQR) | 75 (69–80) | 73 (67–80) | 75 (69–80) | 74 (70–79) | 75 (70–79) | 74 (69–78) | 0.7 |
| ECOG-PS, n (%) | |||||||
| 0–1 | 640 (89.4) | 39 (90.7) | 472 (88.9) | 28 (100) | 84 (89.4) | 17 (85.0) | 0.41 |
| ≥2 | 76 (10.6) | 4 (9.3) | 59 (11.1) | 0 (0.0) | 10 (10.6) | 3 (15.0) | |
| Baseline PSA level (ng/mL), median (IQR) | 183.2 (43.7–735.4) | 128.7 (36–504) | 182.4 (46.3–731.6) | 52.7 (21.9–111.8) | 287.5 (69.3–1202) | 392.2 (148.0–1570.7) | <0.001 |
| Gleason score, n (%) | |||||||
| ≤7 | 62 (8.7) | 7 (16.3) | 46 (8.7) | 4 (14.3) | 4 (4.3) | 1 (5.0) | 0.18 |
| 8 | 204 (28.5) | 8 (18.6) | 148 (27.9) | 12 (42.9) | 32 (34.0) | 4 (20.0) | |
| 9 | 373 (52.1) | 24 (55.8) | 276 (52.0) | 12 (42.9) | 50 (53.2) | 11 (55.0) | |
| 10 | 77 (10.8) | 4 (9.3) | 61 (11.5) | 0 (0.0) | 8 (8.5) | 4 (20.0) | |
| Extent of disease, n (%) | |||||||
| Lymph node | 354 (49.4) | 43 (100) | 231 (43.5) | 13 (46.4) | 53 (56.4) | 14 (70.0) | <0.001 |
| Bone | 643 (89.8) | 0 (0.0) | 531 (100) | 0 (0.0) | 94 (100) | 20 (90.0) | <0.001 |
| Lung | 130 (18.2) | 0 (0.0) | 0 (0.0) | 28 (100) | 94 (100) | 8 (40.0) | <0.001 |
| Liver | 17 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 17 (85.0) | <0.001 |
| Other visceral | 4 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (20.0) | <0.001 |
| EOD score, n (%) | |||||||
| 0–1 | 290 | 43 (100) | 189 (35.6) | 28 (100) | 23 (24.5) | 7 (35.0) | <0.001 |
| 2 | 199 (27.8) | 0 (0.0) | 165 (31.1) | 0 (0.0) | 28 (29.8) | 6 (30.0) | |
| 3 | 187 (26.1) | 0 (0.0) | 145 (27.3) | 0 (0.0) | 37 (39.4) | 5 (25.0) | |
| 4 | 40 (5.6) | 0 (0.0) | 32 (6.0) | 0 (0.0) | 6 (6.4) | 2 (10.0) | |
| CHAARTED Criteria, n (%) | |||||||
| High-volume | 533 (74.4) | 0 (0.0) | 391 (73.6) | 28 (100) | 94 (100) | 20 (100) | <0.001 |
| Low-volume | 183 (25.6) | 43 (100) | 140 (26.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hormone therapy, n (%) | |||||||
| ARSI doublet | 336 (46.9) | 13 (30.2) | 259 (48.8) | 11 (39.3) | 45 (47.9) | 8 (40.0) | 0.16 |
| CAB or ADT | 380 (53.1) | 30 (69.8) | 272 (51.2) | 17 (60.7) | 49 (52.1) | 12 (60.0) | |
| Radiation to primary site, n (%) | 45 (6.3) | 9 (20.9) | 34 (6.4) | 1 (3.6) | 0 (0.0) | 1 (2.8) | <0.001 |
3.2
Oncological outcomes
Of all patients, 286 (39.9%) developed CRPC, 183 (25.6%) exhibited disease progression after second-line therapy, 89 (12.4%) suffered cancer-specific deaths, and a total of 143 (20.0%) died. The Kaplan-Meier curves indicated significantly shorter times to CRPC and PFS2 in the M1c _other group ( Fig. 2 ). The castration-resistant prostate cancer-free survival (CRPCFS) Kaplan-Meier curves for the M1a and M1c_lung_only groups almost overlapped, as did the curves for the M1b and M1_lung_bone groups ( Fig. 2 ). Such trends were also observed in patients receiving ARSI doublet therapy ( Fig. 2 ). In contrast, neither the CSS nor OS differed significantly among the groups ( Supplementary Figure 1 ). Multivariate analysis revealed that the M1c_other groups had significantly shorter times to CRPC and PFS2 in both the overall and ARSI cohorts ( Table 2 and Supplementary Table 2 ). Supplementary Tables 3 and 4 present 3-year data for CRPC, PFS2, CSS, and OS, stratified by the metastatic sites of the total and ARSI cohorts, respectively.