Fig. 5.1
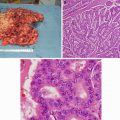
Inflammatory breast cancer: Note the swollen breast, erythema, skin thickening, and peau d’orange. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
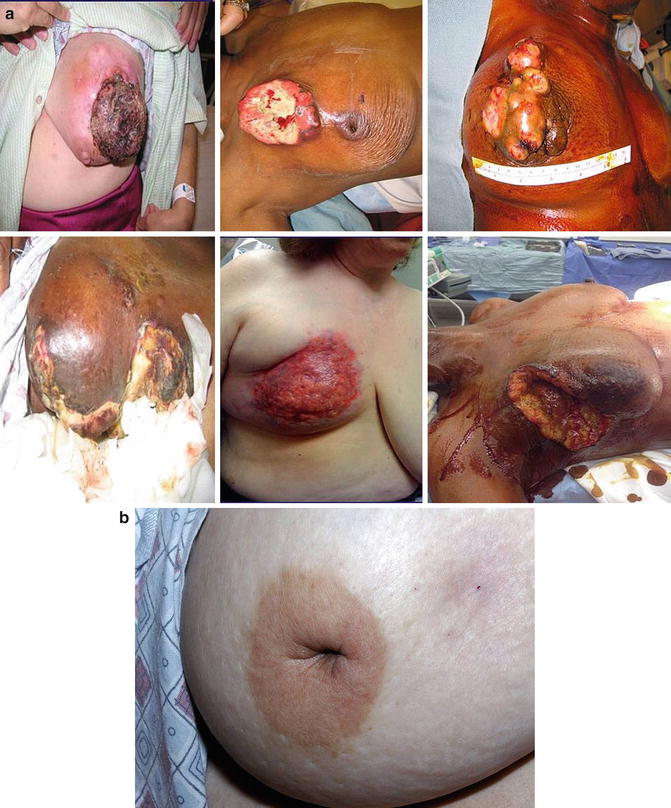
Fig. 5.2
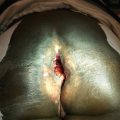
(a): Pictures of patients with non-IBC LABC. (b): A patient with non-IBC LABC who presents with nipple retraction. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Despite their advanced stage at presentation, a considerable percentage of patients with LABC can be cured, and thus, treating physicians should aim for a curative intent when caring for these patients.
Clinical Presentation
Inflammatory Breast Cancer (IBC)
Inflammatory breast cancer (IBC) (T4d in the American Joint Committee on Cancer staging system) represents the most aggressive variant of breast cancer, accounting for approximately 1–5 % of all breast cancers in the United States. Clinically, patients present with a rapid onset of breast tenderness, erythema, edema, pain, skin thickening, and breast swelling. Nipple retraction can occur when the central portion of the breast is involved (Fig. 5.2b). Nearly 55–85 % of patients with IBC have axillary lymph node involvement at presentation and about 30 % have distant disease at the time of diagnosis [3]. The clinical picture is caused by tumor blockage of the lymphatic channels. Such presentation can be mistaken for mastitis or breast abscess (Fig. 5.3), which can contribute to a delay in diagnosis and treatment. In general, mastitis occurs almost exclusively in lactating women. A trial of antibiotics for 1 week with a close follow-up is a reasonable approach for those that present with a low clinical suspicion for IBC. However, failure to have a complete resolution of signs and symptoms should prompt the clinician to proceed with further investigation to rule out IBC.
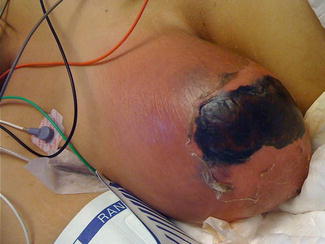

Fig. 5.3
A patient with a breast abscess that mimics IBC. An incision and drainage was performed and a biopsy of the skin and underlying tissue excluded the diagnosis of cancer. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The diagnosis of IBC is primarily clinical with histologic confirmation of invasive carcinoma [4]. Histologically, IBC is diagnosed by showing evidence of tumor cells in the dermal lymphatic channels, although this pathognomonic finding is not necessarily a prerequisite for its diagnosis; dermal lymphatic invasion is found in only approximately 60 % of patients with IBC [5].
Our understanding of the behavior of IBC is limited due to the rare incidence of IBC and our lack of having a clear definition of IBC. In 2008, an international panel of experts convened and developed criteria for IBC, and they are as follows: (1) a rapid onset of breast erythema, edema and/or peau d’orange, and/or warm breast, with or without an underlying palpable mass; (2) duration of history of no more than 6 months; (3) erythema occupying at least one-third of the breast; (4) and histologic confirmation of invasive carcinoma [6].
Non-IBC Locally Advanced Breast Cancer (Non-IBC LABC)
Non-IBC LABC includes large tumors (>5 cm or T3), tumors of any size that involve skin and/or chest wall (cT4a-c or Stage IIIB), tumors with fixed or matted axillary lymph nodes or tumors clinically detected in the ipsilateral internal mammary nodes without involvement of axillary lymph nodes (N2), and tumors that involve ipsilateral infraclavicular, supraclavicular, or internal mammary lymph nodes with axillary lymph nodes involvement (cN3 or Stage IIIC).
On occasion, women with non-IBC LABC can present with an inflammatory recurrence of the chest wall following a mastectomy, but such a presentation is considered as secondary IBC. This is different than primary IBC.
Although IBC and non-IBC LABC are grouped together under the LABC category, they nevertheless represent two distinct biologic entities [7–9]. In contrast to non-IBC LABC, there is a higher incidence of IBC in African-American women; affected women with IBC tend to be younger and have a high body mass index. Biologically, IBC possesses lower ER expression (up to 80 % of IBCs have ER-negative and/or PR-negative tumors [10]), high nuclear grade, high mitotic index, increased tumor expression of E-cadherin, and higher HER-2 expression (up to 50 % of IBC tumors) [11–13]. Furthermore, IBC has a poorer prognosis compared to non-IBC LABC and has the propensity for distant soft-tissue, brain, and bone metastases [4, 7, 8, 14]; patients with IBC have a 43 % increased risk of death from breast cancer compared to patients with non-IBC LABC [8].
Diagnosis and Staging
The initial tests for women with LABC are the same as for those with early breast cancer. A bilateral mammogram is often the first imaging study to be done to detect synchronous lesions in the ipsilateral and contralateral breast (Fig. 5.4). For patients who present with a large, fungating neglected breast carcinoma, an ipsilateral mammogram, which requires compression, may cause unnecessary discomfort for the patient. In such a situation, an ipsilateral mammogram can be avoided since a significant percentage of these patients may not be candidates for BCT. However, when a significant response is observed following neoadjuvant therapy and the patient is a candidate for BCT, a post-chemotherapy mammogram, ultrasound, or MRI should be performed.
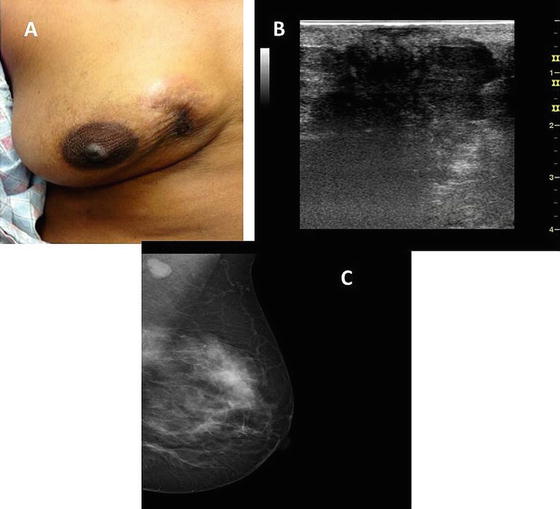

Fig. 5.4
A 60-year-old Caucasian woman with a noninflammatory locally advanced right breast cancer (a). Because of the extent of disease, she was not able to tolerate a right mammogram. However, a right breast ultrasound demonstrated a solid, hypoechoic mass with irregular borders and posterior acoustic shadowing (b). Her left mammogram demonstrated a synchronous breast cancer with an apparent nodal disease (c). (Courtesy of Quyen D. Chu, MD, MBA, FACS)
For patients with non-IBC LABC, a mammographic abnormality such as a mass will be obvious. For those with IBC, a mass may not be present in up to 40 % of patients. The most common mammographic findings for patients with IBC are signs of inflammation, which include skin and trabecular thickening and diffuse opacity (Fig. 5.5). Ultrasound is a useful adjunct to mammography. Besides showing marked skin thickening and edema of the subcutaneous plane, evidenced by diffuse hyperechogenicity and architectural distorting with marked posterior acoustic shadowing [15], ultrasound can help with assessing nodal involvement. Ultrasound-guided biopsy of enlarged lymph nodes can be done. It is reported that ultrasound can detect up to 93 % of ipsilateral axillary nodal involvement and up to 50 % of infraclavicular, supraclavicular, and internal mammary nodal involvement [16].
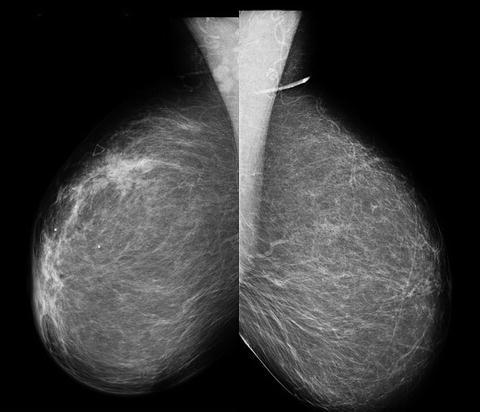

Fig. 5.5
Mammogram of a patient with IBC showing skin thickening and a breast mass (left) in contrast to the normal breast (right) (Courtesy of Stacy Lee, MD, Louisiana State University Health Sciences Center Shreveport)
The role of MRI in patients who clearly have non-IBC LABC is not well characterized. It is probably not necessary, especially for those who will require a mastectomy. However, MRI does have a role when evaluating response to induction chemotherapy in patients with non-IBC LABC (please see subsequent section below). For patients with IBC, MRI may be the most accurate test for detecting a primary breast lesion since imaging features seen in mammograms are not specific for IBC [17] (Fig. 5.6).
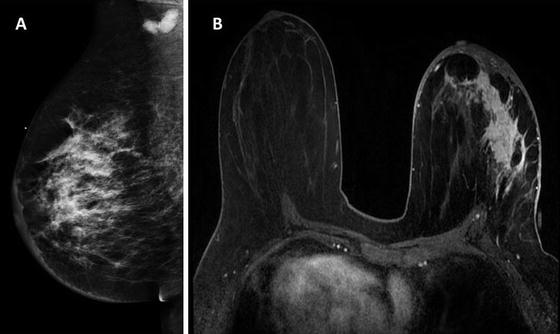

Fig. 5.6
A 48-year-old woman presenting with a three-month history of left breast heaviness. MLO view shows pleomorphic calcifications with associated trabecular and skin thickening suspicious for inflammatory breast cancer (a). Axial T1-weightedf fat-suppression post contrast image shows a large area of confluent enhancement with overlying skin thickening suspicious for malignancy (b). A core biopsy confirmed high-grade invasive ductal cancer and ductal carcinoma in situ (Courtesy of Priscilla Slanetz, MD, Beth Israel Deaconess Medical Center, Harvard Medical School)
For the majority of patients, a core needle biopsy is all that is necessary to establish a diagnosis for patients with IBC and non-IBC LABC; often, sufficient material from the FNA is available to perform assays for hormone and HER-2 receptors according to the American Society of Clinical Oncology/College of American Pathologists guidelines [18–21]. However, if there is insufficient material to establish a diagnosis or obtain receptor statuses, an incisional biopsy may be required. For those with palpable axillary lymph nodes, an FNA can also be performed to confirm evidence of nodal disease. For patients with IBC, preferably two punch biopsies (Fig. 2.3 of Chap. 2) are needed to evaluate for evidence of tumor emboli in the dermal lymphatic channels and confirm the diagnosis of carcinoma (Fig. 5.7). This is helpful to confirm IBC and also in a situation when a core biopsy is inconclusive.
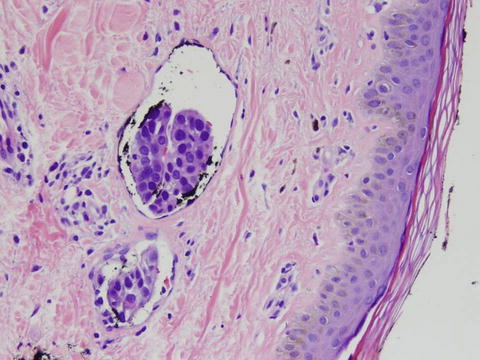

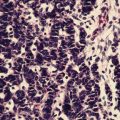
Fig. 5.7
Hematoxylin and eosin stain of an IBC specimen demonstrating dermal tumor emboli in the lymphovascular spaces. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Because patients with LABC are at an increased risk of having concurrent distant disease, a metastatic work-up is often performed, which can include chest X-rays, bone scans, liver ultrasounds, abdominal CT scans, PET/CT scans, and MRI’s, with the intent of identifying patients who have incurable disease and would receive palliative therapy only [22]. The National Comprehensive Cancer Network (NCCN) guidelines recommend a metastatic work-up for patients with T3N1 disease (Stage IIIA), yet for those with N2/N3 disease, a metastatic work-up is considered optional [23]. Chu et al. recently evaluated 256 patients with N2/N3 diseases and demonstrated that T stage is a useful barometer to select patients who might require additional metastatic work-up. For patients with T0, T1, or T2 diseases, the incidence of Stage IV disease was 0 %, 0 %, and 6 %, respectively. However, this incidence increases with higher T stage; 22 % for T3 and 36 % for T4 tumors [22]. Thus, the authors recommend a routine metastatic work-up for patients with N2/N3 diseases who have T3/T4 tumors. Further validation is needed to confirm such findings. A panel of international experts recommend that all patients with IBC should undergo a metastatic work-up with a CT and a bone scan [6].
The use of [18] F-FDG PET scan is considered optional in the work-up of patients with LABC (category 2B in NCCN guidelines) [23]. It is useful in situations where standard imaging studies are equivocal or suspicious [23]. PET/CT outperforms bone scanning such that a positive result on a PET/CT precludes the need to pursue a bone scan [24]. PET scan can also detect additional distant lesions that were not seen on conventional imaging [24] (Fig. 5.8). A prospective study evaluating 117 patients with LABC who underwent conventional imaging methods as well as PET/CT found that PET/CT outperformed conventional imaging methods. PET/CT revealed unsuspected lymph node involvement in 32 additional patients that were missed by conventional imaging modalities. In addition, distant metastases were detected in 43 patients using PET/CT versus 28 patients with conventional imaging modalities. PET/CT altered the stage of 61 patients (52 %), which impacted the recommended treatment for the patients [24]. Whether PET/CT impacts overall survival remains unknown.
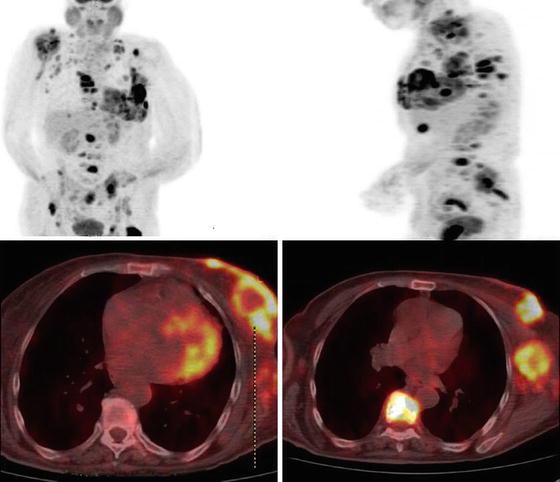

Fig. 5.8
PET/CT scan showing a left locally advanced breast cancer that has widespread distant metastases. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Treatment Overview: Multimodality Approach
Historically, outcome with single modality for patients with LABC has been dismal. When treated with radiation ± chemotherapy, radiation plus surgery, or combined chemoradiation therapy plus surgery, the 5-years DFS was 6 %, 24 %, and 40 %, respectively [26]. Untreated IBC can lead to the demise of more than 90 % of patients within 1 year. More recent data demonstrated that multimodality therapy comprising of chemotherapy, target-specific therapy, surgery, and radiation resulted in 5-years overall survival that can reach greater than 60 % [27].
Unlike early breast cancer, there is a paucity of phase three clinical trials on LABC. Additionally, many trials evaluating LABC grouped IBC and non-IBC LABC together, treating them as one disease rather than as distinct entity. Furthermore, randomized trials of neoadjuvant therapy often include a mix bag of patients that included patients with large operable breast cancer as well as those with IBC and/or non-IBC LABC. Finally, the duration of treatment and sequence of agents is undefined for patients with LABC. Regardless of these limitations, there are some general principles that currently hold true when managing patients with LABC. For one, neoadjuvant chemotherapy (NAC) comprising of anthracyclines (i.e., Adriamycin, epirubicin) and taxanes (i.e., paclitaxel, docetaxel) is used for both IBC and non-IBC LABC. Conventional wisdom dictates that concomitant HER-2-targeted therapy (trastuzumab) with anthracyclines should be avoided due to cardiotoxicity. If trastuzumab is to be used for those with HER-2-positive tumors, a non-anthracycline regimen of docetaxel and carboplatin has been recommended. However, recent data suggest that combination of trastuzumab and anthracycline is relatively safe and effective [28] (see section below). If trastuzumab is to be used, it should be given in conjunction with neoadjuvant chemotherapy and continued postoperatively for a total of 1 year.
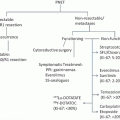
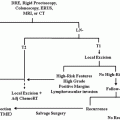
Four to six cycles of preoperative systemic therapy should be considered over a course of 4–6 months before surgery [6]. For patients with IBC who had a clinical response (partial or complete) with neoadjuvant therapy, a modified radical mastectomy (MRM) should be performed, followed by postoperative radiation and hormonal therapy, if indicated [23]. Breast conserving therapy (BCT) is an option for patients with non-IBC LABC who had a clinical response. However, for those who did not respond to neoadjuvant therapy, taxane should be administered for those that are taxane naïve, and radiation should be offered prior to an MRM (Fig. 5.9). Local control rates when surgery is performed following neoadjuvant therapy for nonresponders, partial responders, and complete responders are 33 %, 68 %, and 89 %, respectively [29]. Although controversial, a delay in reconstruction in patients with LABC should be considered. However, some patients may strongly desire immediate reconstruction. Skin-sparing mastectomy is contraindicated in patients with IBC.
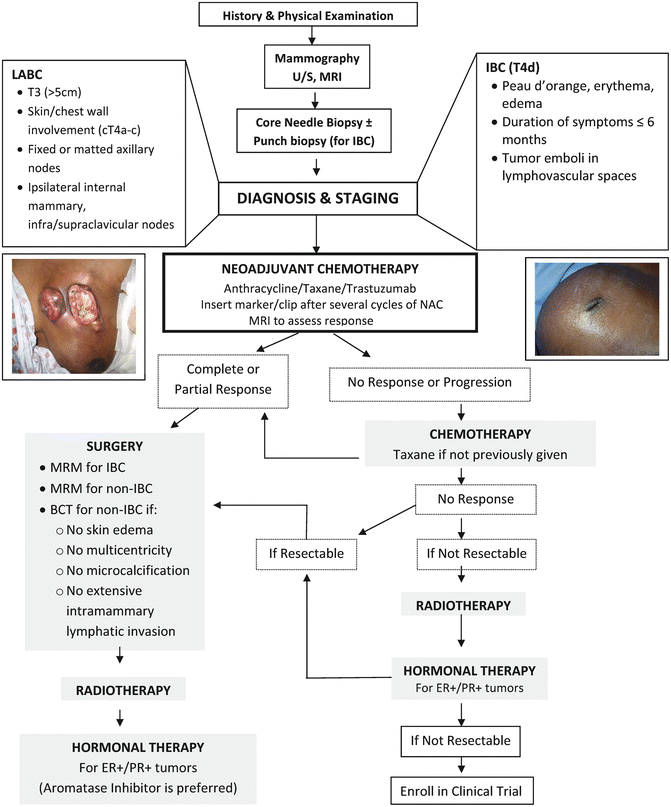

Fig. 5.9
Algorithm for the diagnosis and treatment of patients with LABC. (Courtesy of Quyen D. Chu, MD, MBA, FACS)
In general, adjuvant chemotherapy (i.e., chemotherapy following surgery) is not necessary if the patient had completed her scheduled NAC regimen. Surgery in the form BCT is an option for select patients with non-IBC LABC, while it is contraindicated for those with IBC. Although there are occasional reports of success with BCT in patients with IBC, BCT should only be performed in a clinical trial.
Radiation is an integral part of managing patients with LABC, irrespective of the degree of tumor response to NAC. Radiation fields should encompass supraclavicular and internal mammary lymph nodes for patients. Figure 5.9 shows an algorithm for the diagnosis and treatment of patients with LABC.
cCR and pCR Definition
Patients undergoing NAC are followed closely to evaluate their response to therapy. Clinical assessment of response to therapy relies on palpating the lesion to determine whether the primary tumor has decreased in size and if so, whether it is a partial clinical response (residual palpable mass) or a complete clinical response (cCR; no palpable mass). Although a cCR can be achieved, this does not necessary mean that there is an absence of residual tumor on the final pathologic specimen; it is possible for a tumor to achieve a cCR but not a pathologic complete response (pCR) (i.e., on pathologic sectioning, viable tumor cells are seen; this is referred to as achieving partial pathologic response or pPR). However, if there is no residual tumor seen under the microscope, the patient is deemed to have a pathologic complete response or pCR. Conversely, a patient may have an abnormality as seen on imaging or clinical exam following neoadjuvant therapy, yet has pCR. This can occur in a third of patients. What this means is that regardless of the sensitivity of imaging and clinical examination following NAC, a surgical specimen is still needed for determining the final pathologic response status.
The rates of cCR and pCR vary among the series and are dependent on the regimen used. In general, about 27 % of patients will achieve cCR and 10–26 % for pCR [30, 31]. pCR rate for anthracycline is less than 15 %, but this rate increases to 33 % with the addition of taxane and can reach up to 55 % with the addition of trastuzumab [32].
Standardized definition of pCR is lacking; pCR can mean (1) no pathologic residual tumor in the breast tumor, (2) no invasive component in the breast and axillary nodes, (3) complete absence of invasive and noninvasive component (DCIS), or (4) acceptable to have presence of focal invasive cancer or noninvasive residual tumor (i.e., DCIS) in the final specimen [33]. The American Society of Clinical Oncology guidelines define pCR as total disappearance of malignant cells, both in the breast and axillary lymph nodes [34].
pCR is a surrogate marker for long-term outcomes [31, 33–35]. Patients who achieve pCR have more favorable outcomes than those who do not. This is applicable for patients with IBC as well as noninflammatory LABC. In a study of 61 patients with IBC by Hennessy et al, the 5-year DFS was 78.6 % for those who had pCR, but dropped to 25.4 % for those with residual disease. Similarly, the 5-year OS was 82.5 % in the pCR group while it was 37.1 % in those with residual disease [36]. A recent meta-analysis of 12 randomized controlled trials of neoadjuvant systemic therapy demonstrated that pCR from both the breast and the lymph nodes was associated with improved event-free survival (HR = 0.48; P < 0.001) and overall survival (HR = 0.36; P < 0.001) [31]. Elevated Ki-67, a marker of proliferation, before NAC was found to be an independent predictor of pCR in LABC [37, 38]. pCR is accepted by the FDA as an endpoint for approving novel medication for patients with high-risk tumors. Whether pCR translates to an improved long-term outcome will need to be proven by randomized trials [33].
pCR status is a paradox; the more aggressive subtypes (i.e., triple-negative breast cancer or TNBC and HER-2+/nonluminal) tend to carry a poorer prognosis compared to the less aggressive subtypes (i.e., luminal A and B), yet they are more likely to achieve pCR status. In fact, a greater pCR rate is seen not only in the TNBC and HER-2+ tumors but also in those that are ER negative, poorly differentiated, and highly proliferative [31, 33]. Cortazar et al. found that pCR rates range between 7 and 16 % in hormone receptor-positive (HR+) tumors (i.e., less aggressive types), 18–30 % in HR+/HER2+ tumors, 31–50 % in HR-/HER2+ tumors, and 34 % in TNBC [31].
Although pCR is a useful prognostic marker for the more aggressive subtypes (i.e., TNBC and HER-2+), it may not play such an important role in the more favorable subtypes. For instance, because of the excellent prognosis that is often associated with luminal A subtype, pCR status has very little or no impact on outcome. Conversely, for patients with the aggressive subtypes such as TNBC or HER-2+/nonluminal, pCR is highly prognostic; those that achieved pCR in these subgroups have outcome similar to those that have the luminal A subtype [33].
For patients who achieved pCR following neoadjuvant chemotherapy, whether postmastectomy radiotherapy (PMRT) should be given remains an area of intense investigation. In a study of 106 patients (84 % had LABC) who achieved a pCR (no evidence of invasive carcinoma in the breast or axillary lymph nodes), the 10-year local-regional recurrence (LRR) rates with and without PMRT were 7.3 % and 33.3 %, respectively (P = .04). PMRT was associated with improved disease-specific and OS, even in those who achieved pCR [39]. However, in another study of 134 patients with Stage II–III breast cancer, PMRT did not have an impact on LRR or OS in those who achieved pathologically negative LNs following neoadjuvant chemotherapy [40]. Despite these compelling data, most clinicians would opt for PMRT for patients with LABC.
A multidisciplinary panel of expert, under the auspice of the National Cancer Institute (NCI), recommends PMRT following neoadjuvant chemotherapy for patients presenting with clinical Stage III disease or those with positive lymph nodes after neoadjuvant therapy. As an aside, for those who initially present with Stage II disease who achieved pCR following neoadjuvant therapy, there is limited data to support PMRT routine use [41]. National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials showed that the 8-year risk of locoregional recurrence (LRR) after a mastectomy for patients who initially present with clinical Stage II disease is less than 10 % for those with negative LNs after preoperative chemotherapy [41].
Neoadjuvant Chemotherapy (NAC)
The two major goals of treating patients with LABC are to obtain locoregional control and eradicate occult systemic disease. Central to such goals is neoadjuvant chemotherapy. Neoadjuvant chemotherapy, also referred to as preoperative chemotherapy, induction chemotherapy, or primary chemotherapy, allows downsizing and downstaging of large inoperable primary tumors so that they can become operable. NAC also increases BCT rate, provides insight to the effectiveness of the chemotherapeutic regimen, answers research questions, and allows earlier treatment of micrometastatic disease (Fig. 5.10a, b).
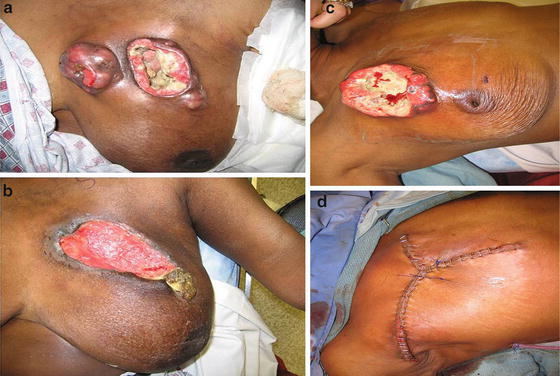

Fig. 5.10
A patient with a partial response to neoadjuvant chemotherapy (a, b). Another patient who had a minimal response after optimal NAC and radiation therapy (c, d). The patient underwent a successful modified radical mastectomy without the need for a skin or tissue flap. This was accomplished using the “Mercedes-Benz” closure (c). (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The theoretical disadvantages associated with the NAC approach is that the initial tumor size and number of involved lymph nodes may not be accurately assessed. Because of bulky disease, NAC will have to treat a much greater disease burden. There is also a concern of an increased risk for surgical complications and drug resistance. Because surgery is performed later, there is a potential delay in curative local therapy should the patient not respond to NAC [42–44]. Despite these disadvantages, the advantages with the neoadjuvant approach appear to outweigh the risks. Regardless, there are no significant differences in OS or disease progression between patients undergoing NAC or adjuvant chemotherapy [45]. These facts were demonstrated in the NSABP B18 (4 cycles of Adriamycin and cyclophosphamide (AC) before versus after surgery) and NSABP-B27 (similar regimen as B-18, except taxane was added) trials and confirmed by a meta-analysis of nine randomized studies [45]. It should be noted that since there is no phase III data comparing NAC with adjuvant chemotherapy in patients with LABC, the assumption that NAC is comparable to adjuvant chemotherapy is based on retrospective studies [46] as well as on data extrapolated from operable breast cancer.
Most clinicians caring for patients with LABC would opt for the NAC approach rather than the adjuvant, and thus, NAC has become the standard of care for patients with LABC. Up to 90 % of patients undergoing NAC achieved major objective responses and downstaging. Following NAC, surgery alone, radiotherapy alone, or a combination of both modalities has been reported for non-IBC LABC patients. For patients with IBC, an MRM is the surgery of choice. Surgical options for non-IBC LABC include an MRM or a lumpectomy, axillary lymph node dissection, and postoperative radiotherapy. Patients who do not want the NAC approach and have resectable disease should undergo surgery (usually an MRM) followed by adjuvant chemoradiation therapy.
All patients undergoing NAC should have a fiducial clip, marker, or small coil inserted inside the tumor prior to completion of NAC. This is especially important because up to 30 % of patients undergoing NAC will have pCR, with the implication that there may not be any detectable or palpable disease to help guide the surgeon when he/she proceeds with excision. A marker may not be necessary for patients with IBC since the majority of them will receive an MRM.
For patients with LABC, multiple studies demonstrated that anthracycline-containing regimen resulted in superior response rate over non-anthracycline regimen [47]. The addition of a taxane to systemic anthracycline-based regimen further refined the neoadjuvant strategy [48–51] (Table 5.1). NSABP-B27 enrolled 2,411 patients with operable breast cancer that included clinical T1–3, N1, M0 to preoperative AC followed by surgery, AC + docetaxel followed by surgery, or AC followed by surgery, followed by docetaxel. The study demonstrated that the addition of docetaxel to the conventional AC regimen in the preoperative setting nearly doubled the percentage of patients achieving a pCR from 13.7 % to 26.1 % (P < 0.001) and increased the percentage of patients achieving a cCR from 40.1 % to 63.6 % (P < 0.001) [48]. Although disease-free survival (DFS) was improved, overall survival (OS) was not with the addition of docetaxel. The Aberdeen Breast Group also reported similar results in a phase III trials of 162 patients with LABC [50]. The pCR was 34 % in the docetaxel group versus 16 % (P = 0.04) in the cyclophosphamide, vincristine, doxorubicin, prednisolone (CVAP) group, and the cCR was 94 % in the docetaxel group versus 66 % in the CVAP group (P = 0.001). Interesting, unlike NSABP B-27, the Aberdeen group demonstrated an improved OS with the addition of docetaxel [50].
Table 5.1
Neoadjuvant chemotherapy for inflammatory and locally advanced breast cancer (non-trastuzumab based)
Study | Year | Patients | Population | Chemotherapy regimen | pCR rate | Overall RR | 5-year DFS | 5-year OS |
|---|---|---|---|---|---|---|---|---|
Hortobagyi [113] | 1988 | 174 | LABC | FAC | 16.7 % | NR | 84 % (for IIIa), 33 % (for IIIb) | 84 %(for IIIa), 44 % (for IIIb) |
Perloff [114] | 1988 | 113 | LABC and IBC | CAFVP | NR | 72 % | NR | NR |
1992 | 107 | LABC and IBC | CAFM | 29 % | 57 % | NR | 61 % (for IIIa), 36 % (for IBC), 31 % (for non-IBC IIIb) | |
2004 | ||||||||
Colozza [117] | 1996 | 31 | LABC and IBC | CAP | 8 % | 76.7 % | 29 % (6 years) | 28 % (6 years) |
Ueno [47] | 1997 | 172 | IBC | FAC, FACVP, FACVP ± MV | NR, NR, NR | 74 % | 32 % | 40 % |
Clark [118] | 1998 | 34 | LABC | A | 21 % | 65 % | 77 % (3 years) | 88 % (3 years) |
Kuerer [35] | 1999 | 372 | LABC | FAC | 12 % | NR | NR | NR |
Cristofanilli [119] | 2001 | 42 | IBC | FAC ± T | 14 % | 81 % | NR | NR |
Favret [120] | 2001 | 64 | LABC | FAC | NR | 92 % | 58 % | 75 % |
Smith [50] | 2002 | 50, 47 | LABC | CVAP, CVAP + docetaxel | 16 %, 34 % | 66 % | 77 % (3 years), 90 % (3 years) | 84 % (3 years), 97 % (3 years) |
Hutcheon [121] | 94 % | |||||||
Harris [122] | 2003 | 54 | IBC | CMF or FAC | 30 % | 52 % | 49 % | 56 % |
McIntosh [123] | 2003 | 166 | LABC | CVAP | 15 % | 75 % | NR | NR |
Therasse [124] | 2003 | 448 | LABC | CEF, EC + filgrastim | 14 %, 20 % | 79.9 %, 85.7 % | NR, NR | 53 %, 51 % |
Espinosa [125] | 2004 | 51 | LABC and IBC | ET | 18 % | 78 % | NR | NR |
Ezzat [126] | 2004 | 126 | LABC | PC | 16 % | 91 % | 63 % | 85 % |
Gajdos [127] | 2004 | 138 | LABC | CMF or CAF | 13 % | 53 % | 46 % | 55 % |
Lebowitz [128] | 2004 | 30 | LABC | Docetaxel + capecitabine | 10 % | 90 % | NR | NR |
de Matteis [129] | 2004 | 30 | LABC + IBC | ET | 13.3 % | 76.7 % | NR | NR |
Shen [130] | 2004 | 33 | LABC + IBC | NR | 12 % | 85 % | 70 % | 78 % |
Thomas [131] | 2004 | 193 | LABC | VACP | 12.2 % | 83.4 % | 51 % | 60 % |
Erol [132] | 2005 | 74 | LABC | CMF | 18.9 % | 88 % | 52 % | 79.9 % |
Gradishar [133] | 2005 | 45 | LABC | Docetaxel | 10 % | 49 % | NR | 80 % |
Kao [134] | 2005 | 15 | LABC and IBC | T + vinorelbine + XRT | 46.7 % | 93 % | 33 % (4 years) | 56 % (4 years) |
Tham [135] | 2005 | 51 | LABC | Docetaxel | 20 % | 75 % | NR | 78 % (2 years) |
Veyret [136] | 2006 | 102 | IBC | FEC-HD | 14.7 | 91.1 % | 35.7 % (10 years) | 41.2 % (10 years) |
Ellis [137] | 2006 | 265 | LABC and IBC | AC + T, metronomic AC + T | 17 %, 26 % | NR, NR | NR, NR | NR, NR |
Villman [138] | 2007 | 41 | LABC and IBC | ECX | 19 % | 74 % | NR | NR |
von Minckwitz [49] | 2008 | 1,390 | LABC and IBC | TAC | 22.2 % | 100 % | NR | NR |
Manga [139] | 2009 | 60 | LABC and IBC | ATX | 8.3 % | 77 % | 76 % (3 years) | 90 % (3 years) |
In a retrospective analysis of 240 patients with IBC, those who were treated with 5-fluorouracil, Adriamycin, cyclophosphamide (FAC) followed by paclitaxel had an objective response rate of 84 % vs 74 % in those with FAC alone. Additionally, the pCR was 25 % for those that received paclitaxel vs 10 % for those with FAC alone. The addition of paclitaxel resulted in an improvement in median OS and progression-free survival as compared to FAC alone [51].
Dose-dense chemotherapy (administration of a full dose of drugs over a shorter time period than standard) data for LABC are encouraging, but is still relegated as investigational and not recommended outside the confines of a clinical trial.
Neoadjuvant Endocrine Therapy (NET)
The role of neoadjuvant endocrine therapy (NET) is not as well defined as that for NAC. Retrospective data suggest that NET may be just as effective as neoadjuvant chemotherapy in terms of achieving clinical response and rate of breast conservation [52]. A phase III trial comparing neoadjuvant chemotherapy versus tamoxifen and GnRHa in women with hormone receptor-positive, HER-2-negative, lymph node-positive, primary breast cancer in premenopausal women (NEST trial; NCT01622361) is being conducted and is expected to be completed in 2016 [53].
Preliminary studies suggest that neoadjuvant aromatase inhibitors (AIs) may be more effective than tamoxifen in decreasing tumor size and increasing breast conservation rate [54–58] (Table 5.2). Whether anastrozole, exemestane, or letrozole is the preferred neoadjuvant AI might not make much of a difference since American College of Surgeons Oncology Group (ACOSOG) Z1031 trial found that the response rates among the three agents were equivalent [59]. Recall that AIs are only effective in women without functioning ovaries (i.e., postmenopausal women) (see Chap. 4, Early Breast Cancer for more thorough explanation). Most of the NET trials were done on postmenopausal women [55–57, 60], except for one, which treated premenopausal patients with goserelin to suppress their ovarian function [58]. Of the five selected trials, only one did not find any significant difference in response rate or breast conservation rate between anastrozole and tamoxifen [58]. Outside of a clinical trial, neoadjuvant endocrine therapy should not be used in young women [61]. Hormonal therapy should only be restricted to patients with hormone receptor-positive tumors.
Table 5.2
Selected clinical trials comparing neoadjuvant aromatase inhibitors with tamoxifen
Authors (Yr) | N | Menopause status | Response rate | BCT rate | ||||
|---|---|---|---|---|---|---|---|---|
Ellis 2001 [55] | 324 | Postmenopausal | Letrozole | Tamoxifen | Letrozole | Tamoxifen | ||
60 % | 41 % | P = 0.004 | 48 % | 36 % | P = 0.036 | |||
Eiermann 2001 [57] | 337 | Postmenopausal | Letrozole | Tamoxifen | Letrozole | Tamoxifen | ||
55 % | 36 % | P < 0.001 | 45 % | 35 % | P = 0.022 | |||
Smith 2005 [60] | 330 | Postmenopausal | Anastrozole | Tamoxifen | Anastrozole | Tamoxifen | ||
IMPACT trial | 58 % | 22 % | P = 0.18 | 44 % | 31 % | P = 0.23 | ||
Cataliotti 2006 [56] | 451 | Premenopausal | Anastrozole | Tamoxifen | Anastrozole | Tamoxifen | ||
PROACT trial | 39.5 % | 35.4 %b | P = 0.03 | 43 % | 30.8 %
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||