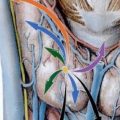
Fig. 9.1
(a) Axial CT image during the arterial phase of contrast medium enhancement demonstrates a brightly enhancing 8 mm diameter nodule (arrow) in the anterior portion of the pancreatic head consistent with an insulinoma. (b) Axial CT image in the same patient demonstrates a second brightly enhancing 3 mm diameter nodule (arrow) in the pancreatic tail. (c) Single image from coeliac axis arteriogram demonstrates both tumor nodules (arrows). Arterial stimulation venous sampling performed during the same procedure confirmed that the pancreatic head lesion secreted insulin and that the tail lesion was nonfunctioning. The patient went on to have a curative laparoscopic enucleation of the pancreatic head tumor
With a biochemical diagnosis of an insulinoma in whom the CT shows the typical appearances of a single intrapancreatic neuroendocrine tumor, there is a good argument for referring the patient for surgical resection at this stage without further imaging although endoscopic ultrasound may still provide important additional information such as the relationship of the tumor to the pancreatic duct.
Despite recent advances in MDCT, there will still be a significant number of patients in whom a tumor will not be visualized, and these individuals will need further investigation.
Endoscopic Ultrasound (EUS)
In experienced hands, this investigation is recognized as being one of the most sensitive modalities for the detection of small intrapancreatic neoplasms with detection rates in several series of over 90 %.
The entire pancreas can be examined in the majority of individuals; the body and tail of the pancreas are imaged through the gastric wall and the pancreatic head via the duodenum. High-frequency transducers (8–12 MHz) are used, and tumors as small as 5 mm may be detected.
Most tumors will be markedly hypervascular on color Doppler ultrasound, and tumor conspicuity may be further enhanced by using intravenous “bubble” contrast medium.
Fine-needle aspiration cytology can also be performed although this is rarely necessary if a single neoplasm is seen in the context of hyperinsulinemic hypoglycemia.
Magnetic Resonance (MR) Imaging
As with CT, a meticulous scanning technique, including bowel paralysis, is important, and intravenous enhancement with gadolinium may be required. Tumors are usually of low signal intensity on T1-weighted images, especially if fat suppression sequences are used, and will show enhancement following intravenous contrast medium. On T2-weighted images, tumors are more likely to be hyper- or isointense when compared with the normal surrounding pancreatic parenchyma.
The reported sensitivity for the detection of primary pancreatic insulinomas varies considerably from as low as 20 % to one approaching 100 %; a figure of between 50 and 70 % is probably reasonable.
MR will not infrequently demonstrate tumors which have not been localized on MDCT particularly in patients with MEN1. Those patients in whom there is biochemical evidence of a functioning insulinoma, but have multiple pancreatic neoplasms, will usually require further investigation (often angiography combined with arterial stimulation venous sampling) if surgical excision is being considered to try and determine which of the tumors is functioning.
Radionuclide Imaging
Radiolabeled Somatostatin Analogs
A variety of tumors, both neuroendocrine and non-neuroendocrine, contain somatostatin receptors (SSTr). NETs frequently express a high density of SSTr, particularly the SSTr2 subtype, which forms the molecular basis for somatostatin receptor scintigraphy. Somatostatin receptor scintigraphy (SRS) with 111 Indium-DTPA-octreotide (111In-octreotide) has proved to be highly sensitive in localizing and documenting the extent of disease in the majority of these neoplasms with reported sensitivities of 75–100 %. The one exception is insulinomas for which the reported sensitivities are 40–60 % due to their lower incidence of somatostatin receptors in general and of the subtype 2 in particular.
Positron Emission Tomography (PET) Combined with CT (PET-CT)
PET using 18-fluorodeoxyglucose (FDG), a glucose analog, has become very useful in general oncology but has a limited role in the investigation of pancreatic neuroendocrine tumors and, in particular, insulinomas due to the relatively slow metabolic rate of NET cells.
More recently, 68Gallium-DOTA peptide PET imaging has shown greater promise in the documentation of disease extent for both low- and high-grade GEP tumors, and there is recent evidence that it, and other newer PET isotopes, may help localize small insulinomas (Fig. 9.2).
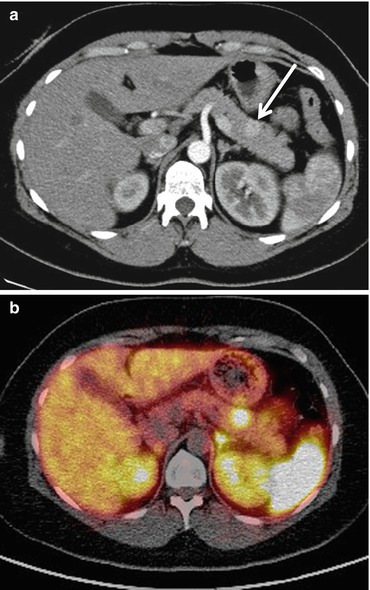
Fig. 9.2
(a) Axial CT image during the arterial phase of contrast medium enhancement demonstrates a 12 mm diameter brightly enhancing tumor in the pancreatic body (arrow) consistent with an insulinoma. (b) Single fused image from 68Gallium DOTATATE PET-CT study demonstrates intense uptake of the radionuclide within the tumor. No other abnormal uptake was seen. The patient went on to have curative laparoscopic resection
In general, 68Gallium-DOTA peptide PET imaging has superseded SRS with 111In-octreotide due to its higher binding affinity and different receptor profile with resultant greater accumulation of radiotracer in SSTr positive cells. Furthermore, the higher spatial resolution of PET images when compared with those obtained by SPECT permits the detection of smaller lesions. There are other advantages:
The study is completed in 2 h while 111In-octreotide imaging requires longer acquisition times and is performed over 2 days.
The tracer is cheaper as it is generator produced.
Unfortunately, however, 68Gallium-DOTA peptide PET imaging is currently not widely available largely due to regulatory issues regarding manufacturing authorization; this should be overcome in the near future.
Angiography and Arterial Stimulation Venous Sampling
Visceral angiography with arterial stimulation venous sampling (ASVS) used to be considered the most sensitive investigation for the detection of insulinomas but is now less frequently necessary. The indications for its use are:
When the other investigations described above have failed to identify an intrapancreatic neoplasm
When there is more than one intrapancreatic neoplasm and information is required about function to direct localized surgical resection (Fig. 9.1c)
Localization of an insulinoma by ASVS relies upon a detectable rise in insulin in hepatic venous samples after the selective injection of calcium gluconate in turn into the arteries supplying different portions of the pancreas. It only localizes the tumor to a region of the pancreas rather than to a specific site if the angiogram does not demonstrate a tumor blush. It has the advantage, however, of being able to confirm that a visualized angiographic abnormality is a functioning tumor.
Functioning Gastrinomas
Sixty percent of gastrinomas are multicentric or have metastasized at the time of diagnosis and 40 % are extrapancreatic, most commonly within the duodenum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree