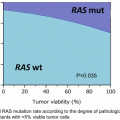
T1N0
No evidence of lymphovascular invasion
Well-differentiated to moderately differentiated tumor
Tumor < 3 cm in diameter
Involved < 30 % circumference of rectal wall
Posterior Approach
The approach to locally resect rectal cancer can be grouped into a posterior approach and the transanal approach (Table 17.2). For the posterior approach, Kraske posterior proctotomy [7] and York-Mason transsphincteric approach [22] are established procedures. However, they are not frequently used nowadays because of the associated complications (sacral pain after coccygectomy, rectal fistulas, and impairment of sphincter function), the fear of seeding tumor in the retrorectal space, the concern of compromising salvage resection, and the advent of newer and less invasive procedures [23, 24].
Table 17.2
Approaches for local excision of rectal adenocarcinoma
Posterior approach |
Kraske transsacral proctotomy |
York-Mason transsphincteric approach |
Transanal excision |
Park’s per anal excision |
Transanal endoscopic microsurgery (TEM) |
With endoscopic posterior mesorectal excision |
With intraperitoneal anastomosis |
With two-stage total mesorectal excision |
Transanal minimally invasive surgery (TAMIS) |
Robotic-TEM |
Regardless, the approach is briefly mentioned for completeness. The patient is placed in the prone position, an incision is made over the sacrum to the upper border of the sphincter and the coccyx, and the anococcygeal ligament is removed to facilitate exposure. To obtain exposure of the upper rectum, the lower margin of the gluteus maximus muscle and sacrotuberous and sacrospinous ligaments are cut, and the lower most part of the left wing of the sacrum is excised (the classical excision of the lower sacrum is not practiced). The levator muscle is incised in the midline to expose the posterior aspect of the rectum. Proximal dissection is carried along the presacral fascia posteriorly and laterally all the way posterior to the prostate gland. The fascia propria of the rectum is incised, and the mesorectum is divided to the rectal wall that is opened transversely and the lesion is excised. With transsphincteric approach, the sphincter complex is divided, and the cut edges are marked with sutures for precise repair.
Transanal Local Excision (TLE)
Parks’ per anal excision [15] [transanal local excision (TLE)] describes excision of tumors under direct visualization through the anal orifice. The procedure is limited to small tumors (<3–4 cm or <30 % of the circumference of the rectal lumen) that are not fixed to the levator muscle.
The procedure is performed under general or spinal and local anesthesia (Fig. 17.1). The patient is place in a jackknife position or on stirrups depending on the location of the tumor. Using various retractors, the lesion is assessed, and the tissues surrounding the tumor are infiltrated with epinephrine-containing solution. Traction sutures may be applied at the lateral or superior edge of the tumor to help prolapse the lesion. The lesion is excised with the Bovie cautery to include a 1 cm circumferential mucosal margin and deep into the perirectal fat (full thickness disc excision). The surgical specimen is oriented for the pathologist. The resulting defect is closed transversely with simple interrupted sutures. For anterior lesions, care must be taken not to injure the vagina. Excision using endoscopic linear stapler-cutter does not reliably excise the full thickness of bowel wall. Morbidity of TLE is minimal and usually related to bleeding or urinary retention.
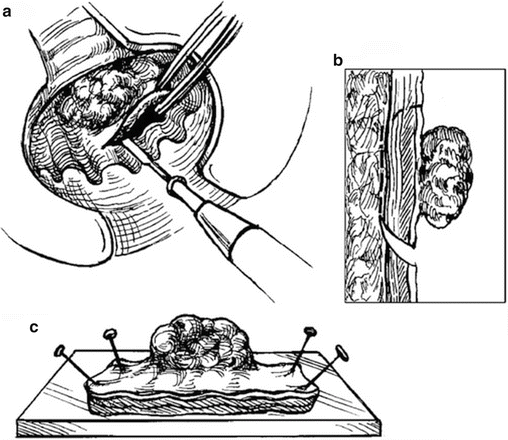

Fig. 17.1
Depiction of transanal local excision (TLE) (Reprinted from Beck et al. [186]. With permission from Springer Verlag)
Transanal Endoscopic Microsurgery (TEM)
Transanal endoscopic microsurgery (TEM) describes transanal local excision using specialized equipment that allows for clear and magnified visualization of the rectal lumen and facilitates dissection and removal of larger lesions located higher up in the rectum that are not amenable to be removed by TLE (up to 20 cm from the anal verge) [25, 26] (Fig. 17.2). Compared to TLE, TEM allows exploration of perirectal fat and regional LN basin, provides superior quality of resection, prevents fragmentation of the specimen, and minimizes margin positivity [27]. The tumor is excised, and dissection is performed with a spatula, hook, or needle point tip monopolar cautery. A 0.5–1 cm margin with pyramidal volumetric excision of perirectal fat for possible retrieval of regional LN is performed.
Initially, TEM was considered inappropriate for advanced neoplastic lesions and lesions beyond the peritoneal reflection for risk of peritoneal breach and contamination. However, repair of the defect prevents conversion to an open procedure and is not associated with major complication or oncological compromise [28–30]. With larger and more locally advanced lesions, segmental resection with end-to-end anastomosis has been described [31–33].
TEM was developed in early 1980s in Tubingen, Germany, by Dr. Gerald Buess in collaboration with the Richard Wolf Medical Instruments company [25, 26]. The equipment for TEM is prepackaged and includes all that is necessary to perform the surgery: operating proctoscope (4 cm diameter, 12–20 mm long, beveled or straight end, and removable faceplate), angled camera that provides a 3-dimensional stereoscopic vision, and carbon dioxide insufflator. A faceplate with clear window is attached for initial insertion and positioning of the proctoscope over the tumor and then replaced with another faceplate for carrying out the procedure. The faceplate is airtight and has four ports sealed by capped rubber/silastic sleeves and accommodates 3 flexible channels [2 working instruments (5 mm long handle instruments for dissection, excision, and suturing) and one suction irrigation instrument] and one optical stereoscope (binocular eyepiece that provides precise 3-dimentional view of the operative field with up to sixfold magnification). At least 3 mm negative deep and mucosal margins are required. After excision of the lesion, the defect is closed, but for the extraperitoneal rectum, the defect may be left opened.
TEM has been used with other techniques to widen the extent of resection. TEM excision of lower third T1 rectal cancer with mesorectal excision using dorsoposterior extraperitoneal pelviscopy has been described [34, 35]. Also TEM excision of the primary tumor and a two-stage TME have been described as sphincter-saving procedures for locally advanced ultralow rectal cancer after neoCRT [36]. However, long-term oncologic outcome from such a procedure is not known, and the approach is not widely accepted.
TEM is widely accepted in Europe but is used only in select centers in the United States. The equipment is expensive, and the procedure is technically challenging requiring a long learning curve [37].
Transanal Minimally Invasive Surgery (TAMIS)
Transanal minimally invasive surgery (TMIS) describes transanal excision of rectal tumors using laparoscopic instruments, traditional insufflation device, and a platform adapted from laparoscopic single-port device (Fig. 17.3). It is essentially a hybrid between TEM and single-port laparoscopy. A 5 mm port for the assistant and a flexible endoscope are used to improve functionality and access and visualization of the rectal lumen as high as 15 cm [38–40]. Compared to TEM, the equipment is less expensive, the device is easier to place, setup time is quicker, and operative time is faster [41].
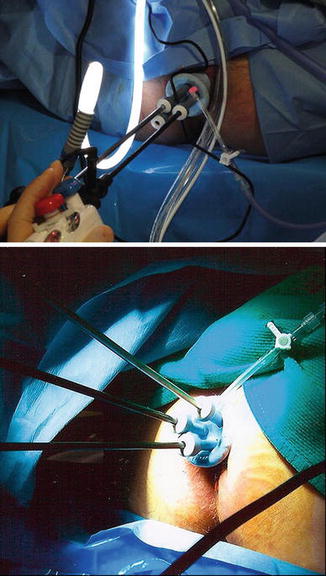

Fig. 17.3
Transanal minimally invasive surgery (TAMIS) (Reprinted from Atallah et al. [39]. With permission from Springer Verlag)
Robotic Transanal Endoscopic Microsurgery (Robotic-TEM)
Robotic-TEM describes TEM using customized “glove port” fitted to circular anal dilator and the da Vinci system that allows maneuverability in deep spaces to overcome some technical difficulties (limited mobility of laparoscopic instruments) with TEM and TAMIS [41–44] (Fig. 17.4). Furthermore, an experienced camera operator is not required. Compared to TEM, the learning curve is shorter [37].
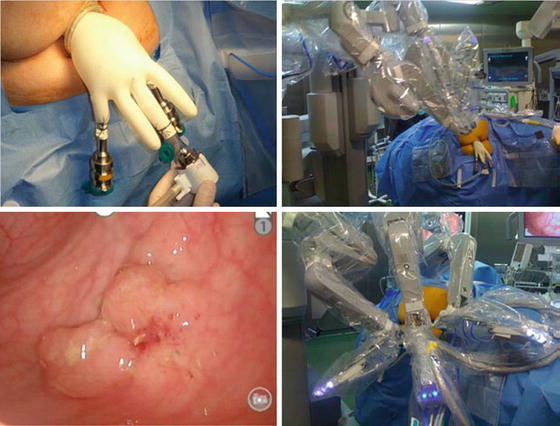

Fig. 17.4
Robotic transanal endoscopic microsurgery (Robotic-TEM) (Reprinted from Valls [183]. With permission from Springer Verlag)
Selection Criteria for Local Excision of Rectal Adenocarcinoma
Surgical treatment of rectal cancer depends on the location of the tumor. Cancers of the upper rectum behave like colon cancer and are treated with anterior resection. Middle and distal third cancers are treated with low anterior resection and mesorectal excision with or without reconstruction or AP resection for lesions too distal to permit an anterior resection. Select cases of rectal cancer, especially distal rectal cancer, may be treated with local excision with or without adjuvant therapy. Thus, the discussion of local treatment of rectal cancer is primarily focused on distal rectal tumors.
Selecting patients for local excision is based on patient factors, location, and clinical stage of the tumor as determined by a digital rectal examination (DRE) and endoscopy, staging radiological studies, and endoscopic biopsies and at the time of excisional biopsy results. The ideal candidates for “curative” local excision are those whose tumors are confined to the rectal wall, more likely to be free of LN involvement and less likely to locally recur. These strict clinical criteria, however, remain imperfect, and surgeons therefore must be vigilant in their follow-up of these patients.
Examination
The initial assessment of the patient includes a history and physical examination, a DRE, and a proctoscopic examination. The patient’s medical conditions (comorbidities), physical limitations, and anal control are assessed. On DRE, the size, morphology, location, and mobility of the tumor and its distance from the anal verge are assessed. On proctoscopy, characteristics of the tumor such as its gross appearance, whether it is ulcerated or polypoid; its dimensions; its distance from the anal verge or dentate line; and its relationship to anatomical luminal landmarks are determined as well as a tissue biopsy for diagnosis is obtained.
Tumor size predicts LR and LN involvement. The risk of LN involvement is 29 % for lesions <2 cm, 17–31 % for lesions <3 cm, and 43–50 % for lesions <4 cm [45–47]. In a recent study, tumor size with a threshold of 34 mm has been suggested to complement clinical staging of patients with rectal cancer [48]
Ulcerated lesions are associated with a greater failure rate and increased risk for LN involvement when compared to polypoid lesions, 49 % versus 11 % [45, 48, 49] (Fig. 17.5). It is advisable that patients with such a characteristic and without contraindication are best considered for radical surgery.
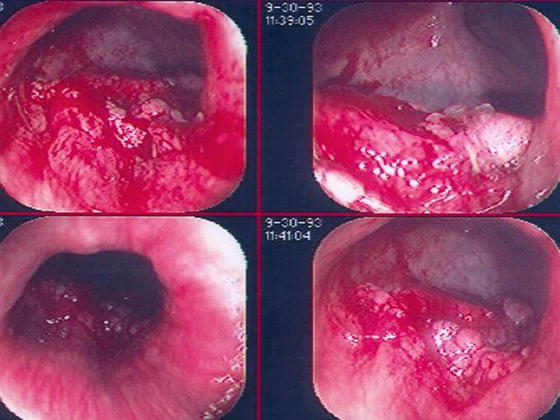

Fig. 17.5
Small distal rectal lesions not amenable to local rectal excision. Ulcerated, bleeding small distal rectal cancer should undergo radical surgery due to a greater failure rate and risk of lymph node involvement
Radiological Studies
Endorectal Ultrasound (ERUS)
ERUS has the ability to delineate the layers of rectal wall and identifies enlarged regional LN. Based on the depth of invasion and LN involvement, the tumor is classified into uT0 to uT4 and uN0 or uN1 (Table 17.3) [50].
Table 17.3
Ultrasound staging of rectal adenocarcinoma
uT0 – tumor confined to the mucosa |
uT1 – tumor confined to the mucosa and submucosa |
uT2 – tumor penetrates into but not through the muscularis propria |
uT3 – tumor extends into the perirectal fat |
uT4 – tumor extends into the adjacent structure |
uN0-N1 – absence or presence of suspicious metastatic LN |
The accuracy of ERUS is operator dependent and varies with pathological stage of the primary tumor and quality of the scanner used (length of the probe, 7 vs. 10 MHz, and resolution). It is superior to conventional CT scan and MRI in detecting LN. ERUS does not determine whether the enlarged LN is inflammatory or neoplastic and does not assess the involvement of the mesorectum or the mesorectal envelope. Unlike the 2-D ERUS, 3-D ERUS has the ability to demonstrate spatial relationship of the tumor in the transverse, sagittal, and coronal planes and determine distance to anal sphincter and is better at differentiating LN from blood vessels [51, 52]. Compared to 2-D ERUS, the accuracy of 3-D ERUS for T stage is 85–91 % versus 83–90 % and for N stage is 76–88 % versus 67–83 % respectively [51–55].
Magnetic Resonance Imaging (MRI)
MRI is more accurate than CT scan at assessing the circumferential radial margin (CRM). With the use of the endorectal coil (EMRI), the accuracy of MRI approaches that of ERUS. The accuracy of EMRI for T and N staging is 85 % and 82 %, respectively, compared to 87 % and 74 %, respectively for ERUS. When compared to CT scans, the accuracy of EMRI for T and N staging is 82 % and 74 %, respectively, and 73 % and 66 % for CT scan [56–60]. However, the wide use of MRI is prohibitive in many centers because of its high cost.
Since up to 50 % of positive LNs in the mesorectum are <1 cm in size, LN staging by size criterion alone is inaccurate and understages the cancer. Spiculated or indistinct border and a mottled heterogeneous appearance are new criteria used to increase the accuracy of predicting LN involvement [61]. The 3 tesla MRI accurately predicts tumor depth and CRM involvement as well as reliably stages the LNs by morphologically evaluating it and the blood vessels within the mesorectum [62–64]. Regardless of the imaging modality being used, accurate staging remains imperfect. This may partly explain the sobering recurrence rate of 10–29 % for T1 and 26–47 % for T2 cancers following local excision [65–67].
Computerized Tomography Scan (CT Scan)
CT scan is useful in detecting distant metastasis and tumor extension into adjacent organs, but it is inadequate for T staging of ESRC since it does not delineate the layers of the rectal wall. Additionally, CT scan is limited in its ability to detect regional LN. The accuracy for T staging is 46–75 % and for LN staging is 56–72 % [58, 68].
Positron Emission Tomography Scan (PET Scan)
PET scan can help detect locoregional and distant spread and has been used to determine response to chemoradiation therapy (CRT) in patients with advanced rectal cancer. Its role in staging the cancer preoperatively is yet to be determined, although some studies have shown that PET scan alters management in up to a third of patients.
Histopathological Features of the Primary Tumor
The histopathological features of the primary tumor are helpful in selecting patients who may be candidates for local excision. Such features are determined from endoscopic biopsies or after complete removal of the tumor (Table 17.4). These features are discussed in details in the following section, and recommendations assumed that the patient is a surgical candidate for cure. Of note, the decision for definitive operation is easily made when many or all of the histological features are available from the initial biopsy. However, in situations where some or all of the features are known only after a definitive local rectal excision has been performed, the surgeon must consider counseling the patient to undergo a definitive operation within a thirty-day window period [69].
Table 17.4
Histopathological features important in selecting patients for local excision of rectal adenocarcinoma
Histological type |
Grade |
Depth of invasion |
Presence or absence of |
Blood vessel invasion |
Perineural invasion |
Angiolymphatic invasion |
Lymphocytic infiltration |
Increased tumor infiltration with lymphocytes |
Extramural lymphocytosis |
Infiltration in the stroma in the region of tumor and lymphoid nodules often in the region of the tumor edge |
Infiltration at the edge of the muscularis propria analogous to Crohn’s Disease |
Budding at edge of tumor |
Surgery-related factors: |
Completeness of excision |
Fragmentation |
Tumor Type
The World Health Organization (WHO) classification of rectal cancer is depicted in Table 17.5 with the most common carcinoma being adenocarcinoma [70].
Table 17.5
Histologic classification of rectal cancer
Adenocarcinoma |
Mucinous adenocarcinoma (>50 % mucinous) |
Signet cell adenocarcinoma (>50 % signet cell) |
Small cell (oat cell) carcinoma |
Squamous cell carcinoma (epidermoid) |
Adenosquamous carcinoma |
Medullary carcinoma |
Undifferentiated carcinoma |
Others (e.g., papillary carcinoma) |
Tumor type is not an independent prognostic factor, except for signet cell and small cell carcinomas. Small cell carcinoma is a high-grade carcinoma with neuroendocrine differentiation that presents with higher stage and has dismal prognosis even with early lesions than non-signet cell carcinomas [71–73]. Local rectal excision must not be performed with these histologies.
Grade
The College of American Pathologists recommends a two-tier classification scheme of rectal cancer: low-grade (well-differentiated and moderately differentiated) and high-grade (poorly differentiated and undifferentiated) carcinomas [74]. Grade of the tumor is a stage-independent prognostic factor with high-grade predicting LN metastasis and adverse outcome [74–77].
Lymph node involvement in well-differentiated to moderately differentiated adenocarcinomas is 0–30 % compared to 50–69 % for poorly differentiated carcinomas [74, 78]. Lymph node involvement is <10 % for T2 well-differentiated adenocarcinoma without adverse histological features compared to 70 % for poorly differentiated with lymphovascular invasion [79]. Thus, patients with high-grade histology are not candidates for local excision.
Depth of Tumor Invasion
The primary tumor in rectal cancer is classified into Tx to T4 depending on the depth of invasion into the rectal wall (Table 17.6) [71]. On occasion, a malignant polyp is found following a routine lower endoscopic examination. T1 malignant polyps are further classified into substages depending on the extent of invasion into the polyp (Haggitt classification) [80] (Fig. 17.6) or into the submucosa (Kikuchi classification) [81] (Fig. 17.7). The Haggitt classification applies mainly for pedunculated polyps with levels of invasion ranging from 0 to 4. The Haggitt classification is as follows: level 0, carcinoma in situ; level 1, invasion of the submucosa but limited to the head of the polyp; level 2, invasion extending into the neck of the polyp; level 3, invasion into any part of the stalk; and level 4, invasion beyond the stalk but above the muscularis propria. By definition, sessile polyps are classified as Haggitt level 4. However, not all sessile polyps behave the same. Because Haggitt classification does not take this into account, the Kikuchi classification of sessile polyp becomes a useful tool to further identify patients who are candidate for local excision. Essentially, Kikuchi classification divides the submucosa layer into thirds, Sm1 through Sm3.
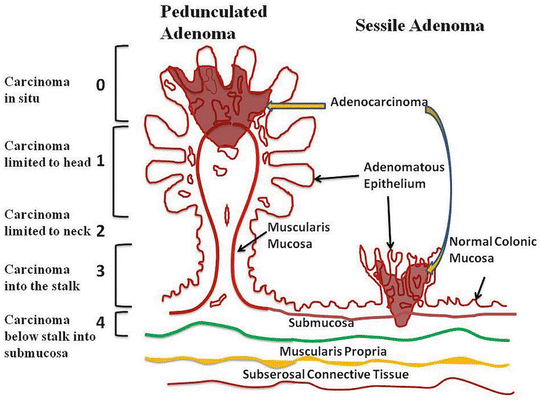
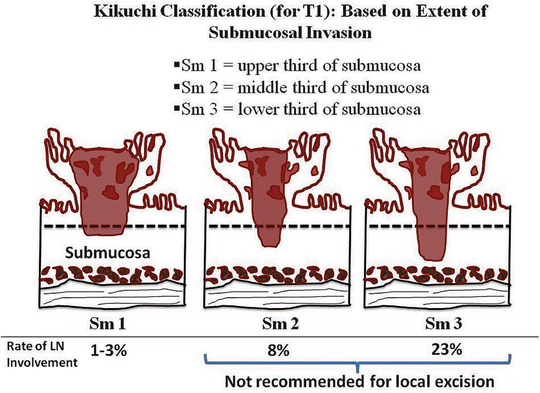
Table 17.6
T staging of colorectal adenocarcinoma
Tx = primary tumor cannot be assessed |
T0 = no evidence of primary |
Tis = carcinoma in situ (intraepithelial invasion of the lamina propria or muscularis mucosae) |
High-grade dysplasia = no stromal invasion |
Intramucosal carcinoma = stromal invasion identified |
T1 = tumor invades into the submucosa |
T2 = tumor invades into the muscularis propria |
T3 = tumor invades through the muscularis propria into the subserosa or into nonperitonealized pericolic or perirectal fat (pT3a-3d) |
T4 = tumor directly invading other organs or structure T4a or perforates the visceral peritoneum T4b |

Fig. 17.6
Haggitt classification. Level 0, carcinoma in situ; level 1, invasion of the submucosa but limited to the head of the polyp; level 2, invasion extending into the neck of the polyp; level 3, invasion into any part of the stalk; and level 4, invasion beyond the stalk but above the muscularis propria (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 17.7
Kikuchi levels. Sm1, invasion of superficial third of the submucosa; Sm2, invasion of middle third of the submucosa; and Sm3, invasion of deep third of the submucosa (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The risk of LN involvement is related to T stage. Carcinoma in situ is not a “true carcinoma” and has no risk of LN metastasis; patients with carcinoma in situ only require wide excision with a negative margin. The risk of LN involvement based on T stage is as follows: T1 = 5–25 %, T2 = 20–28 %, T3 = 36–67 %, and T4 = 53 % [78, 82–89]. The risk of LN metastasis is <1 % for Haggitt levels 1–3 and 12–25 % for level 4 [80, 86, 87, 90]. The depth of the submucosal invasion is related to LN metastasis with none found if invasion is less than 1,075 μm [91, 92]. For pedunculated polyps, LN metastasis is zero for invasive cancer confined to the head and neck and for those where the stalk invasion is less than 3,000 μm without evidence of lymphatic invasion [92, 93]. Lymph node metastasis increases with Kikuchi level: 1–3 % for Sm1, 8 % for Sm2, and 23 % for Sm3 [81, 87, 94].
Angiolymphatic Invasion
Lymphatic invasion is associated with increased regional LN metastasis particularly with pT1 and superficial pT2 lesions [39, 80, 95–100]. Invasion of extramural large vessel in the perirectal fat and outside the muscularis propria is associated with a predisposition for hepatic metastasis. Angiolymphatic invasion is a stage-independent prognostic factor and identifies patients at increased risk for LN metastasis and diverse outcome [76, 101, 102]. Patients with angiolymphatic invasion are not candidates or local excision.
Neural Invasion
Tumor Budding
This refers to microscopic clusters of undifferentiated cancer cells located ahead of the invasive front of a well-differentiated to moderately differentiated tumor. It has greater prognostic value than overall grade and predicts regional LN metastasis in APR specimens of T1 and superficial T2 rectal cancers [97, 104]. Patients with this feature are not considered for local rectal excision.
Host Lymphoid Response to Tumor
LN Involvement
Perirectal LNs include mesorectal, lateral sacral, presacral, sacral promontory, and superior, middle, and inferior rectal LNs. None of these LNs are included in TLE but may be included in the TEM or TAMIS. The absence of LN in the surgical specimen limits their predictive value. Perhaps this partly explains [107] the higher recurrence rate seen with TLE when compared to TEM. The presence of LN metastasis is determined on radiological studies and is predicted based on histopathological features of the primary tumor. A high suspicion of LN involvement as determined on preoperative imaging (i.e., ERUS, MRI, CT) must prompt the surgeon and patient to consider a radical operation over local rectal excision.
Surgery-Related Factors
Following anterior or AP resection, margins assessed are proximal, distal, transverse, and circumferential. After local excision, margins assessed are lateral (circumferential around the tumor) and deep. Surgical margins involved by tumor, either microscopically or grossly, are indicative of residual tumor within the operative field, which predispose the patient to having LR. Piecemeal excision (fragmented excision) is an adverse prognostic factor resulting in a higher proportion of local failures [108]. Thus, patients with positive margins after local rectal excision or those whose tumors underwent piecemeal excision must be considered for definitive radical operation.
Candidates (Patient/Tumor) for Local Excision
Local excision is an attractive treatment option since it preserves rectal function, is associated with rapid recovery, low morbidity, and mortality, and does not result in the need for a colostomy. The operation, however, is controversial since there is lack of level I/level II evidence supporting its oncologic equivalency with standard radical surgery [18, 109]. As an aside, prospective clinical trials are classified as being phase I, II, or III. Phase III trials compare new treatments with the best currently available treatment (i.e., the standard treatment) and are considered the highest level of evidence to guide treatment decision. In the literature on local excision of rectal cancer, although there are a number of phase II trials, there are no phase III trials.
Breen et al. [110] summarized the results of retrospective studies and reported a local recurrence (LR) rate of 0–33 % for ERC following local excision. Yet, local excision has been on the rise despite poor locoregional control with and without adjuvant CRT therapy [18, 66, 111, 112]. Local excision of T2 lesions has tripled in the past 25 years, especially for elderly patients who often have multiple comorbidites [18]. Stitzenberg et al. recently confirmed this trend using the National Cancer Data Base [111]. There is also an increasing rate of local excision in patients with advanced rectal cancer who develop complete response after neoCRT where the risk of occult LN is reportedly to be as low as 3–6 % [113–116]. Surgeons must be aware of the limitations of the current available data and be cautious when selecting patients for local rectal excision. When selecting local excision, patient and tumor factors must be considered, and clinical and radiological staging of local tumor is the most important aspect of the selection.
Patient Factors
Patients with anal incontinence and poor mobility are not candidates for a sphincter-saving procedure. On the other hand, those who are unfit or refuse to undergo major abdominal surgery, even with advance disease such as those with ≥T3 or ≥N1, may be considered for local excision for symptomatic relief (bleeding and discharge) [117]. Local excision is also an attractive alternative to radical surgery in patients with recurrent cancer for whom a major operation is prohibitive. This is especially applicable to elderly patients with multiple comorbidities [118].
Tumor Factors
Local excision is restricted to tumors that are confined to but do not involve full thickness rectal wall (T2 or less) or regional LN and have favorable histopathological features. Classically, tumors that are polypoid and not ulcerated, located with proximal margin <10 cm from the anal verge, appeared <3–4 cm in size, encompassed <30 % of luminal circumference of the rectum, and not fixed to the sphincter or puborectalis muscle may be considered for local excision. Conversely, local excision is not appropriate for:
Large lesions encompassing >40 % of rectal circumference, since local excision can lead to a loss of rectal volume and/or stricture formation resulting in poor function
Proximal anterior or lateral lesions since excision is associated with a risk of peritoneal contamination and potential tumor dissemination
Stage III cancer since local excision does not adequately address the LN and nodal involvement is substantial high in this population
However, there is no conclusive evidence that size is a limiting factor in the selection criteria for local excision. A more suitable criterion is the ability to achieve an adequate tumor-free margin. It is thought that adjuvant therapy may improve LR rates after local excision, although these highly selected results are based on uncontrolled studies (i.e., retrospective studies). Reduction in tumor size after neoadjuvant therapy is found to be a reliable prognostic factor of success of local excision [109, 119]. Again, such data are limited due to their retrospective nature. TEM, TAMIS, and Robotic-TEM do not only facilitate the resection of more proximal, larger, and locally advanced lesions but allow for more complex excision/resection. With the advent of effective neoCRT that results in significant or complete response of locally advanced rectal cancer, the use of such modality along with local excision has been on the rise [113, 115, 116, 120, 121]. Whether such a practice yields oncologic equivalence with standard radical resection remains a controversial topic.
As mentioned in the previous section, factors that affect recurrence rate and outcome after local excision of rectal adenocarcinoma include T stage, degree of differentiation, lymphovascular invasion, and positivity of resection margins [67, 78, 94, 109, 122–126]. Local recurrence for ERC, especially those with adverse histopathological features, i.e., high-risk early ERC [poorly differentiated and mucinous adenocarcinoma, signet ring and undifferentiated adenocarcinomas, lymphovascular invasion (absence of lymphoid infiltration and tumor budding are relative factors)], is higher than previously reported [66, 127]. More importantly, the risk of leaving behind nodal disease after local excision of a T2 lesion with adverse histopathological features is 70 % compared to 10 % for T2 with no adverse histopathological features [101, 128].
Hence, local excision alone is ideal for incidental adenocarcinoma following polypectomy, especially for sessile polyp that was incidentally removed and found to have involved margins. It is ideal for Haggitt 1–3 or Kikuchi Sm1 T1 with favorable histological features and T1 lesions that have no adverse features and clear margin. Local excision alone is controversial for Kikuchi Sm2 T1N0 and T2N0.
The following situations require further treatment after local excision:
Positive margins or inadequate tissue for accurate pathological staging histological: patient can be considered for re-excision or proceed with radical surgery.
Radical resection for:
T1 with unfavorable histological features
For T1 Kikuchi Sm3 where risk of LN metastasis mirrors T2
T2
T3 lesions because of high LR rate
Adjuvant therapy has been considered for the following patient population, although radical surgery should seriously be considered:
T1 with unfavorable histopathological features
T2
Higher-risk patient not amenable to more radical surgery
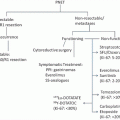
An algorithm for evaluating patients with distal rectal cancer is depicted in Fig. 17.8.
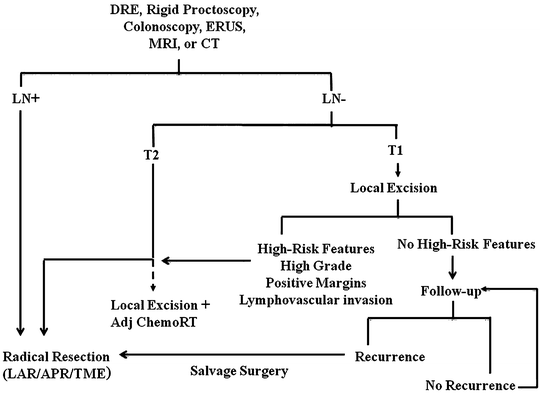

Fig. 17.8
Algorithm for evaluating small distal rectal cancer
Outcome of Local Excision of Rectal Adenocarcinoma
Transanal Local Excision (TLE)
There is great variation in the oncological results following TLE that is mostly related to the number of patients included in the study, patient selection for TLE, and follow-up period after TLE [109]. With short-term follow-up, local excision is associated with a favorable outcome, 10–13 % LR [18, 66, 77, 82, 83, 110, 112, 129]. With long-term follow-up, however, the risk of LR is higher 15–30 %, 12–18 % for T1 and 28–47 % for T2, and recent studies have shown even higher recurrence rate [65–67, 130–135]. The pattern of first recurrence is predominantly local, 50 % LR only, 18 % local and distant, and 32 % distant [66, 130]. Local recurrence after local excision develops quickly, whereas distal recurrence is delayed [67]. The overall recurrence (local + systemic) is higher with TLE compared to radical resection, 21 % versus 0% for T1 and 47 % versus 16 % for T2 [65, 131, 132]. With radical resection, failure is mostly distal with LR occurring in 6–10 % [136, 137].
Bhangu et al. [138] reported the survival outcome of TLE (n = 7,378) versus radical resection (n = 36,116) of colon and rectal cancer in a Surveillance, Epidemiology, and End Results (SEER)-based study in 2013. Cancer survival rates for carcinoma in situ and T1 rectal cancer were not significantly different but reduced for T2 rectal cancer. In a nationwide cohort study from the National Cancer Data Base, 2124 patients with T1 and T2 diagnosed with rectal cancer between 1994 and 1996 were treated with TLE (n = 765) and standard surgery (n = 1,359) [18]. The 5-year LR rate in the TLE group was 12.5 % for T1 and 22.1 % for T2 compared to 6.9 % for T1 and 15.1 % for T2 in the standard surgery group. The 5-year OS rate in the TLE group was 77.4 % for T1 and 67.6 % for T2 compared to 69 % for T1 and 76.5 % for T2 in the standard surgery group. For ERC with favorable histology, low LR and high 5-year OS rate approaching 100 % are possible [18, 133, 139]. Since about half of the recurrences are locoregional, radical resection may be curative. However, there is limited data on salvage surgery [65, 133]. Few patients can be treated successfully, at the first clinical recurrence, but the actuarial survival after salvage surgery for LR is low (30–50 %% at 5 years) [65, 66]. In a retrospective study of 155 patients, Baron et al. reported definite survival advantage with immediate radical surgery. The 5-year DFS was 94 % compared to 55 % for delayed salvage [140].
Transanal Local Excision and Adjuvant Therapy
Since LR in patients with T2 cancers treated with TLE is high, it is thought that adjuvant therapy may reduce the risk of recurrence. Minsky [141] noted that with local excision and postoperative RT, local failure was 3 % for T1, 10 % for T2, and 24 % for T3. Fortunato et al. [142] reported less favorable outcome in 21 patients (mostly T2 lesions) with 19 % LR that occurred up to 48 months following resection and 19 % of patients developing distant metastasis; the 5-year DFS was 59 %. Paty et al. [66] noted that RT delayed LR (median 2.1 years vs. 1.1 years for LE only), but overall rates of local and overall recurrence and survival rates were similar to patients not receiving RT. Thus, postoperative RT had no impact on recurrences.
In a phase II multi-institutional trial of TLE or TLE + adjuvant CRT, the overall recurrence rate at 3 years for T1 or T2 was 4 % [143]. In another prospective study of patients with T1 treated with TLE and T2 with TLE and adjuvant CRT, no T2 patient recurred [144]. In an initial prospective phase II study by the Radiation Therapy Oncology Group, low-grade T1 tumors with negative margins were observed, and CRT or RT was administered based on post-excision results. Local recurrence rates were 7 %, 8 %, and 23 % for T1, T2, and T3 tumors, respectively [145]. Recent data suggest that the recurrence rate for T1, especially those with unfavorable histopathological features, was high and that neoCRT may be administered to sterilize the regional LN, especially when further surgery is not contemplated [128, 139, 146]. The results of other studies are depicted in Table 17.7.
Table 17.7
Studies evaluating outcome of transanal local excision with and without adjuvant therapy
Study | Treatment | Local recurrence |
|---|---|---|
CALBG [182] | T1 = TLE (n =59) | T1 = 7 % |
T2 = TLE (n = 51) | T2 = 14 % | |
T1 and T2 | 6-year OS = 85 % | |
6-year DFS = 78 % | ||
MEDLINE database | ||
Retrospective review, 41 studies [109] | TLE | T1 = 9.7 % (0–24 %) |
T2 = 25 % (0–67 %) | ||
T3 = 38 % (0–100 %) | ||
TLE + adjuvant CRT | T1 = 9.5 % (0–50 %) | |
T2 = 16.6 % (0–24 %) | ||
T3 = 18.8 % (0–50 %) | ||
National Cancer Data Base [18] | TLE: | |
T1 (n = 601) | 12.5 % | |
T2 (n = 164) | 22.1 % | |
Standard surgery: | ||
T1 (n = 493) | 6.9 % | |
T2 (n = 866) | 15.1 % |
TEM and TAMIS
There is a paucity of well-designed studies comparing TLE to TEM, but TEM is superior in terms of visualizing and resecting more proximal lesions and may have better oncologic outcome [147]. Moore et al., in a systematic review of all TEM over 22 years (including 55 case series and 3 comparative studies), noted that TEM is superior to Park’s per anal excision with a 6 % local recurrence rate for TME versus 22 % for Park’s; TEM was more likely to yield negative margins (90 % vs. 71 %) and non-fragmented specimen (94 % vs. 65 %) [148]. Compared to radical surgery, TEM has significantly shorter operating time, lower blood loss, shorter hospital stay, and lower perioperative analgesia requirement [26, 27, 149]. Early and late complications of TEM patients are similar or lower than those undergoing open radical surgery [149–151]. Complication rate is low <10 % (ranges from 6 % to 31 %) and complications are minor [26, 27, 152–156]. Perioperative complications that require converting to laparotomy are hemorrhage and peritoneal entry. However, primary repair of the peritoneal entry can prevent the need to convert to open procedure and is not associated with major complications or oncological compromise [28, 29].
It should be noted that evidence to support TEM is based on case series and retrospective studies, which mostly focused on the technical aspect of the procedure, individual selection criteria, and its value in comparison with TLE and radical resection rather than on its oncologic impact.
T1N0
For T1 cancer, local failure of 0–13 % has been reported with TEM [99, 100, 153, 154, 157]. However, Doornebosch and colleagues, in a systematic review of T1 lesions treated with TEM, noted LR varied between 4 % and 33 % [156]. In a series of 88 patients who underwent TEM for T1 rectal cancer, 18 patients (20.5 %) had LR [158]. Local recurrence rates depend on margin positivity, tumor grade, status of lymphovascular invasion, and muscle penetration (T2) [122–126]. The recurrence rate for low-risk T1 is 6 % and for high-risk T1 is 39 %, and immediate operation for high-risk T1 lesions results in a reduction of LR to 6 % [126].
Carefully selected patients have excellent survival, low recurrence rate, and outcomes comparable to radical resection [149, 159–162]. Lee and colleagues retrospectively compared the outcomes of 74 patients with TEM to 100 patients with radical surgery [162]. For T1 cancer, there was no difference in 5-year recurrence or survival between the two groups, but for T2 lesions, there was a higher recurrence rate for the TEM group, although OS was similar for both. In a systematic review of 55 case series, one randomized study and two nonrandomized comparative studies, Middleton et al. noted there was no difference in the recurrence or survival rates or complication rates between TEM and radical resection [27]. In a meta-analysis of 5 studies (one prospective and 4 retrospective, nonrandomized; total of 397; T1 rectal cancer), Wu et al. [163] noted that patients with T1 cancer who received TEM (n = 216) had higher rates of local or distant metastasis at 40 months’ follow-up but with no significant difference in the 5-year OS rate compared to patients receiving radical surgery (n = 181). In another meta-analysis of 11 studies (three randomized controlled, one prospective, 7 retrospective; total of 1,191 patients; T1 and T2) in which 514 patients received TEM, 386 TAE, and 291 standard resection, Sgourakis et al. found that for T1 tumors, local and overall recurrence rates were significantly higher for TEMS versus standard surgery, but there was no significant difference in the distant metastasis rate or OS rate between the groups [164].
The outcome of salvage surgery depends on the timing of surgery. Baatrup et al. reported the outcome of 143 consecutive individuals (multicenter study) treated with TEM for rectal cancer, curative in 43 % and palliative in 5 % [165]. Immediate reoperation was performed in 15 % of cases. Cancer-specific survival for T1 was 94 % [165]. In a series of 88 patients who underwent TEM for T1 rectal cancer, 18 patients developed local recurrence, and of those, 16 underwent salvage surgery with a 3-year OS rate of 31 % and cancer-related survival of 58 % [156].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree