External Beam Radiotherapy
External beam radiotherapy is a useful option in advanced DTC, particularly when the disease is iodine nonavid. It is most commonly used to treat surgically unresectable locoregional disease, as an adjunct to surgery if pathology shows high-risk disease with extranodal extension or soft tissue invasion, or to shrink surgically unresectable disease that threatens important local structures (trachea, larynx, esophagus, or great vessels).
Most data shows benefit to EBRT in the treatment of high-risk incompletely resected locoregional disease [27–29]. Several older studies, however, suggested lack of efficacy, and this has led to controversy regarding its utility [30, 31]. Much of the available data is taken from institutional case series, with varying selection criteria; many of the studies predate the use of intensity-modulated radiation therapy (IMRT) and other newer modalities of radiation that spare more of the normal tissue. Currently, most EBRT is administered via either IMRT or stereotactic body radiotherapy (SBRT). In IMRT, three-dimensional imaging (CT, MRI, and/or PET) is used to determine the size and shape of the radiation beam in order to target the tumor while sparing nearby structures. In SBRT, multiple radiation beams are finely collimated to deliver a higher dose of radiation to a single point, and many fewer sessions are needed [32]. The location of the thyroid poses a particular challenge in the use of EBRT, as tumor and lymph nodes lie in very close proximity to critical structures (such as the aerodigestive system or great vessels) and are at high risk for complications.
Acute toxicities following EBRT include dermatitis, xerostomia, dysphagia, mucositis, hoarseness, dysgeusia, and fatigue. Most are self-limited, although occasionally they may persist. Late effects are more commonly seen with 3DRT versus IMRT. Esophageal stricture may necessitate placement of a temporary or permanent percutaneous endoscopic gastrostomy (PEG) tube. Laryngeal stricture may require temporary or permanent tracheostomy [33, 34]. Late toxicities are rarely seen with IMRT, compared with rates of up to 10% seen with conventional irradiation (3DRT) [35]. EBRT can interfere with healing of recently operated tissues and may exacerbate the xerostomia and respiratory issues caused by radioactive iodine.
Treatment of Metastatic Disease
The incidence of thyroid cancer continues to be on the rise and has increased faster than that of any other solid-organ malignancy. The reported increase is more than 5 % per year in both men and women, partially owing to overdiagnosis attributed to increased imaging of the neck area. The estimated number of new cases for 2016 is 64,300: 14,950 in males and 49,350 in females. Thyroid cancer remains the fifth most common cancer diagnosed in women [36].
While earlier detection has been on the rise, the number of deaths from thyroid cancer has also been increasing with an estimated 1980 deaths per year—910 in males and 1070 in females [36].
Treatment of newly diagnosed DTC aims at a curative approach for this type of cancer. The recurrence risk varies however depending on patient factors, stage at diagnosis, and histologic type. The risk of recurrence is highest for patients whose age is less than 20 or more than 60. Tumors larger than 1.5 cm at diagnosis and those with the presence of extrathyroidal extension also confer a higher risk. The presence of more than five metastatic lymph nodes or clinically apparent N1 disease confers a risk of recurrence greater than 20%. Certain histologic types such as tall cell variant or sclerosing variant have also been associated with an increased recurrence risk [37].
Recurrent Neck Disease
The locoregional recurrence rates for well-differentiated thyroid cancer can vary between 15 and 30% [37–40] and depend partially on the extent of the initial surgery. The decision to observe versus intervene depends on the extent of disease as well as the rate of growth in trying to balance the risks of a second neck surgery.
Small-volume local disease both in the central and lateral neck demonstrates minimal growth in 70–90% of the time [41, 42]. This can be managed conservatively with observation. Bulkier recurrent disease is best managed surgically with a compartment dissection approach of the affected neck even though this may not mean biochemical cure [37, 43]. If metastatic disease is present synchronously, then surgery should only be considered if recurrent local disease is compromising vital structures such as the airway, esophagus, or the recurrent laryngeal nerves [37, 44, 45]. If the disease is unresectable, then EBRT may be an option as previously discussed in this chapter.
Distant Metastases
Even metastatic and radioiodine-refractory DTC can have a long indolent phase whereby the rate of progression of disease does not confer an urgency to initiate treatment [44]. The decision to delay treatment allows patients to continue to lead a good quality of life without the side effects and monitoring schedule of systemic treatment. More importantly, the drugs available to treat such disease—the tyrosine kinase inhibitors (TKIs)—have not yet been shown to prolong overall survival.
In such instances, and if the disease burden does not threaten any vital structures, then the disease can be monitored with serial biochemical and radiologic studies. Tg and thyroglobulin antibody (TgAb) trends can be followed over time. Radioactive iodine scans can also be obtained. In the face of rising Tg and/or TgAb and negative nuclear scans, radioiodine-refractory disease should be considered. In such instances, alternative imaging modalities should be used to monitor radiologic progression of disease, such as comprehensive neck ultrasound and CT scans of the chest. Depending on the clinical scenario, MRI of the brain and/or spine, bone scans, and fluorodeoxyglucose positron emission tomography (18FDG-PET/CT scan) can be considered.
It is important to note that the use of the same imaging modality to monitor progression of disease is paramount. It is also important to use a standardized method for comparison, and the most widely use method at this time is the RECIST (Response Evaluation Criteria in Solid Tumors) criteria. Progressive disease is defined as a 20% increase in target lesion size. Partial response is defined as a 30% decrease in target lesion size. Stable disease is defined as any change in between those values. A comparison of different RECIST criteria versions is listed in Table 21.2.
RECIST 1.0 | RECIST 1.1 | |
|---|---|---|
Target lesions (1) | Measurable target lesions: | Measurable target lesions: |
– Unidimensional measurement | – Unidimensional measurement | |
– ≥20 mm with conventional techniques | – Tumor lesions, ≥20 mm with conventional techniques and ≥10 mm with spiral CT | |
– ≥10 mm with spiral CT | – Malignant lymph nodes, >15 mm | |
– Maximum number: | – Maximum number: | |
– 5 per organ | – 2 per organ | |
– 10 total | – 5 total | |
Nontarget lesions (2) | Size <1 cm, cystic lesions, bone lesions without soft tissue component, serous effusions, leptomeningeal disease, lesions in an irradiated area | Lymph nodes: 10–15 mm |
Non-lymph nodes: Size <1 cm, bone lesions without soft tissue component, serous effusions, leptomeningeal disease | ||
Objective response | 1. CR, disappearance of all target lesions at ≥4 week. PR, ≥30% decrease at 4 week. PD, ≥20% increase or new lesions. SD, neither PR or PD criteria met | 1. CR, disappearance of all target lesions at ≥4 week. PR, ≥30% decrease at 4 week. PD, ≥20% increase and overall 5 mm net increase or new lesions. SD, neither PR or PD criteria met |
2. CR, disappearance of all nontarget lesions and normal tumor markers at ≥4 week. PD, unequivocal progression or new lesions. Non-PD, persistence of ≥1 lesion or abnormal tumor markers | 2. CR, disappearance of all nontarget lesions and normal tumor markers at ≥4 week. PD, unequivocal progression or new lesions or new positive PET scan. Non-PD, persistence of ≥1 lesion or abnormal tumor markers | |
Overall response | – Best response: recorded in measurable disease from start of treatment to progression or recurrence | |
– Non-PD in nontarget lesions reduces CR in target lesions to overall PR | ||
– Unequivocal new lesions are PD | ||
Treatment of distant metastases also depends on the site involved and can further be delineated based on locations as follows:
- 1.
Lung: Lung metastases are the most common site of distant metastases from thyroid cancer. In asymptomatic patients with indolent disease that is not threatening vital structures, serial imaging is recommended. In the setting of localized pulmonary metastases, surgery or EBRT may be considered. If the disease remains radioiodine sensitive, then targeted treatment with I131 radioactive therapy is recommended. However, in refractory disease that is rapidly progressing or threatening vital structures, then consideration for systemic therapy should be given.
- 2.
Bone: The bone is the second most common site for distant metastasis in DTC and is seen in 2–13% of DTC patients [46]. It is often a sign of poor prognosis indicating the widespread and aggressive nature of the disease. They are often symptomatic and confer an increased morbidity and mortality due to skeletal-related events (SREs) such as pathologic fractures, spinal cord compression, and pain. In prior studies of patients with DTC and bone metastases, 51–78% of patients developed at least one SRE [47, 48]. The effective treatment of bone metastases is challenging and consists of RAI therapy if the metastasis is radioiodine sensitive, surgery, and/or EBRT. EBRT is especially considered in instances where the bone metastases are conferring significant pain or threatening a fracture and/or compression. Antiresorptive agents such as bisphosphonates or RANKL inhibitors are also useful in pain control and stabilization.
- 3.
Brain: Brain metastases from thyroid cancer are rare, and the optimal treatment is controversial. Seen in less than 1.5% of thyroid cancers [49], the prognosis is poor, with average survival less than 1 year after diagnosis. I131 radioactive iodine treatment is rarely used and may worsen edema surrounding the metastatic lesion(s), so if it is used, administration of prophylactic steroids must be considered. Solitary lesions may be treated with surgical resection or stereotactic surgery. Extensive disease may be treated with whole-brain radiation therapy.
- 4.
Liver: Liver metastases from DTC are also rare and are seen in around 5% of DTC patients [50]. At the time of discovery of the liver metastases, most patients exhibit other widespread disease, and the treatment in that case is systemic therapy. In some instances, radiofrequency ablation or chemoembolization may be considered [51–53]. Surgical resection may be an option if risks of surgery are acceptable in a patient with widely metastatic disease.
Systemic Therapy
Systemic therapy should be considered in a subset of patients that meet certain criteria for initiation. In asymptomatic patients, progressive disease over a 12-month period, especially if the disease is deemed to be RAI refractory as will be discussed later, is an indication for treatment. Symptomatic patients and patients with bulky disease that threaten vital structures should also be considered when local therapies alone are not enough.
Traditional Chemotherapy
Doxorubicin is the only Food and Drug Administration (FDA)-approved cytotoxic agent for DTC. Response rates however are dismal, and its use in metastatic DTC has become obsolete, especially in the face of the newer targeted TKIs.
Other cytotoxic agents such as paclitaxel, bleomycin, cisplatin, carboplatin, and etoposide have had poor outcomes as well, with minimal (if any) improvement in response rates. Combination chemotherapy with doxorubicin and cisplatin has been shown to increase toxicity without an improvement in response [54, 55].
TKIs
Signaling Pathways
Over the past decade or so, advancements in identifying molecular mechanisms of thyroid cancer tumorigenesis and progression have led to the recognition of specific mutations enabling the development of molecular-targeted therapy in DTC. Elucidating downstream signaling pathways of the Ras/Raf/MAPK and PI3K-AKT pathways has allowed for the identification of oncogenic mutations that have prognostic implications in DTC (Fig. 21.1).
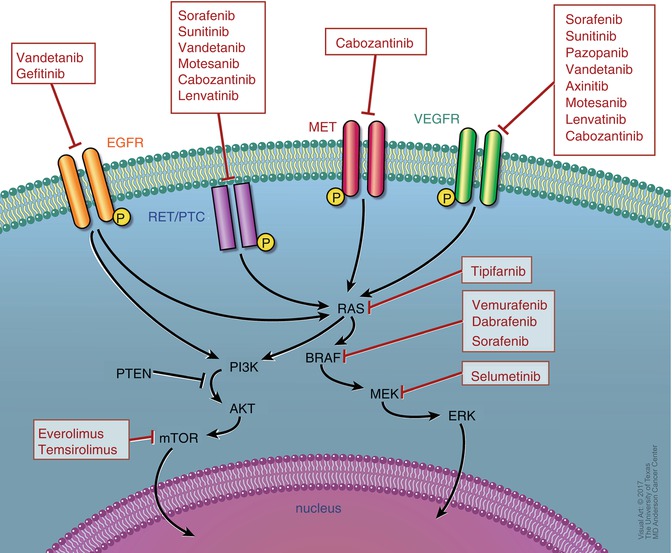

Fig. 21.1
Receptor pathways and targets of clinically available inhibitors
In the MAPK signaling pathway, extracellular signals activate Ras, which in turn activates BRAF. Activated BRAF activates MEK through phosphorylation, and activated MEK in turn phosphorylates ERK to activate it. This leads to intranuclear translocation of activated ERK to regulate transcription of genes involves cell differentiation and survival. BRAF mutations are most commonly seen in DTC and found in up to 40% of primary tumors and 70% of recurrent ones [56, 57]. Among those, BRAF V600E accounts for more than 90% and has been associated with more aggressive and refractory disease [58–61]. RET/PTC and RAS mutations occur in 30–40% of tumors and have also been associated with worse prognosis [62, 63].
In the PI3K-AKT pathway, downstream signaling activates mTOR which is an inhibitor of apoptosis. Angiogenic factors are also upregulated in DTC enabling the use of multitargeted therapies against vascular endothelial growth factor receptors (VEGFRs) and endothelial growth factor receptors (EGFRs) [64–66].
There are currently only two FDA-approved TKIs for DTC, but many others are under investigational trials. The decision to start treatment with TKI should be limited to patients with disease that is either symptomatic or rapidly progressive (growing by at least 20% annually). Consideration should be given to baseline assessment of symptoms prior to initiation of treatment and during therapy [2, 67–69].
Available FDA-Approved TKIs for DTC
Lenvatinib (Lenvima)
Approved in February 2015, 2 months before its goal date, for the treatment of metastatic and progressive DTC that is refractory to radioactive iodine treatment, it is an oral multi-TKI against VEGFR, RET, and FDFR. The recommended starting dose is 24 mg orally once daily.
In the initial multicenter phase II trial, 58 patients with metastatic and progressive DTC were enrolled and received treatment with lenvatinib. Of those, the partial response rate was 50%, and progression-free survival (PFS) was 12.6 months. Subgroup analysis revealed even better PFS rates for patients whose tumors contained a RAS mutation [70].
In the subsequent phase III SELECT trial which was an international, randomized, double-blinded trial, 392 patients with progressive, metastatic, radioiodine-refractory DTC were assigned to receive lenvatinib versus placebo in a 2:1 ratio [71]. The primary endpoint of this trial was PFS, and secondary endpoints evaluated response rates, overall survival, and safety. In the lenvatinib group, the median PFS was 18.3 months versus 3.6 months in the placebo group with a HR of 0.21 (99% CI, 0.14–0.31). This prolongation in PFS was also seen in patients who had been previously treated with one TKI. In terms of response rates, complete response was seen in four patients treated with lenvatinib compared to none in the placebo group. A partial response was noted in 63.2% of patients. Overall survival was not reached, perhaps because patients initially assigned to placebo were allowed to cross over to the treatment group only after demonstration of disease progression [72]. In a subgroup analysis, however, overall survival was reached in the subset of patients older than 65 years of age on treatment with lenvatinib [73].
Most common adverse events seen with lenvatinib were hypertension (68%), fatigue (59%), diarrhea (59%), and loss of appetite (50%). Serious and fatal adverse events, although rare, included thromboembolic events, liver and kidney toxicity, hemorrhagic stroke, and gastrointestinal perforation. The rate of discontinuation of the study drug due to adverse events was estimated at around 14% [72, 74].
Sorafenib (Nexavar)
Approved in 2013 for the treatment of metastatic and progressive DTC that is refractory to radioactive iodine treatment, it is an oral multi-TKI whose targets include BRAF, VEGFR1, and VEGFR2. The recommended daily dose is 400 mg twice a day, to be continued until no clinical benefit is seen or until unacceptable toxicity occurs.
Sorafenib had previously been approved for treatment of renal cell carcinoma (RCC) in 2005 and hepatocellular carcinoma in 2007. Its most recent approval for DTC in 2013 was based on the DECISION trial which was a multicenter, double-blinded, placebo-controlled phase III trial [75]. Four hundred and seventeen patients with locally recurrent or metastatic, progressive DTC refractory to radioactive iodine treatment were enrolled and randomized 1:1 to receive sorafenib 400 mg twice daily or a matched placebo. Baseline characteristics were not different among both groups. Approximately 50% of the patients were male with a median age of 63 (range 24–82). Among the thyroid cancer patients, 57% had papillary, 25% had follicular, and 10% had poorly differentiated carcinomas. Ninety-six percent of all patients had distant metastases, and most common metastatic sites, in order of their occurrence, were the lung (86%), bone (27%), and liver (14%).
The results showed a statistically significant improvement in the median PFS in the sorafenib group (10.8 months) as compared to the placebo group (5.8 months) with a hazard ratio (HR) of 0.59 (95% CI 0.45–0.76; p < 0.0001).
In terms of safety, adverse events occurred in over 98% of the sorafenib group as compared to 88% of the placebo group. There were no serious adverse events, and the most frequent ones observed were hand-foot skin reactions (76%), diarrhea (69%), and rash/desquamation (50%).
There were multiple phase II trials leading up to the DECISION trial that have shown favorable results. The first published phase II trial included 30 patients, 27 of which had DTC [76]. All patients received sorafenib 400 mg twice daily for a median duration of 6.7 months. The partial response (PR) rate by RECIST criteria was 23.3% and stable disease (SD) noted in 53.3% patients. The median PFS in patients who had DTC was 21 months as compared to 18 months in the entry cohort.
Another phase II study included 41 patients with PTC and showed PR in 15% with a median PFS of 15 months [77]. Another long-term outcome study looked at sorafenib treatment for a median period of 9.2 months (range 0.1–39 months) with a median follow-up of 25 months (range 3.5–39 months). Initial response rates were better in the first 6 months of the study as compared to the end of the study with 31% of patients having PR at 6 months compared to 15% only at the end of follow-up [78].
Off-Label TKI Use
After the recognition that a range of tyrosine kinases is involved in the pathogenesis of thyroid cancer, several multitargeted kinase inhibitors are under study in multiple phase I and II trials (Table 21.3). Common targets include VEGFR, PDGFR, and RET. PFS in these trials has ranged from 9.3 to 18 months [79–86].
Table 21.3
Multitargeted kinase inhibitors under study
Drug | Target | Phase | RR (%) | PFS (months) |
|---|---|---|---|---|
Multi-KIs | ||||
Sunitinib | PDGFR, VEGFR, RET, c-kit | II | 31 | 12.8 |
Pazopanib | PDGFR, VEGFR, c-kit | II | 49 | 11.7 |
Vandetanib | RET, VEGFR, EGFR | II | 8 | 11.1 |
Cabozantinib | MET, VEGFR2, RET | I | 53 | – |
BRAF inhibitors | ||||
Dabrafenib | BRAF | I | 29 | 11.3 |
Vemurafenib | BRAF | II | 35 | 15.6 |
Vandetanib (Caprelsa)
Approved for treatment of metastatic unresectable medullary thyroid cancer (MTC), it is a TKI that targets RET, VEGFR, and EGFR. It has been studied in DTC in a phase II trial involving 145 patients with metastatic, radioiodine-refractory disease [83]. Patients were randomized to receive vandetanib 300 mg once daily or placebo and were followed for a median of 19 months. At the end of follow-up, median PFS was 11.1 versus 5.9 months in the vandetanib group as compared to the placebo group (HR 0.63, 95% CI 0.54–0.74). No difference was noted however in partial response rates or overall survival rates. Most commonly observed adverse events were QT prolongation and diarrhea.
Sunitinib (Sutent)
Approved for the treatment of advanced RCC, sunitinib targets multiple VEGFRs as well as RET/PTC subtypes 1 and 3. It has also been studied in open-label phase II trials in patients with progressive DTC at a dose of 50 mg daily for 28 days followed by 14 days of no treatment per cycle. In one study, the partial response rate was 13% [87]. In another study, that rate was 17% [88]. In another trial, sunitinib was given continuously at a dose of 37.5 mg daily. The partial response rate was 25% [89]. Most commonly noted adverse events include fatigue, diarrhea, hand-foot syndrome, and hypertension.
Pazopanib (Votrient)
Also approved for the treatment of advanced RCC, pazopanib is an inhibitor of all VEGFR subtypes only. It has no effect on the RET or BRAF kinases, and its actions in thyroid cancer are limited to its antiangiogenic effects [90]. In a phase II trial, 37 patients with progressive DTC were started on pazopanib 800 mg daily. The partial response rate was 49%, and the PFS rate at 1 year was 47% [82]. The most commonly noted adverse events were hypertension, elevated liver enzymes, and mucositis.
Vemurafenib (Zelboraf)
Approved for the treatment of metastatic melanoma, vemurafenib is a BRAF kinase inhibitor and has been tested in BRAF-positive advanced DTC. In an open-label, multicenter phase II trial, 51 patients with progressive unresectable radioiodine-refractory DTC with a positive BRAF V600E mutation were enrolled and started on vemurafenib 960 mg orally twice daily [86]. There were two cohorts in this study, a TKI-naïve cohort and another cohort whose patients were previously treated with a TKI. The partial response rate in the TKI-naïve cohort was 35% with a median PFS of 15.6 months, while the partial response rate in the previously treated group was 26% with a median PFS of 7 months only. Commonly noted adverse events included rash, fatigue, and alopecia. In another center’s experience, 17 patients received vemurafenib and had a partial response rate of 47% [91].
Dabrafenib (Tafinlar)
Also a BRAF kinase inhibitor approved for the treatment of unresectable metastatic melanoma, dabrafenib has been studied in BRAF-mutated DTC. In a phase I trial of 14 patients with BRAF-mutated DTC, the partial response rate was 29% [85]. This drug has also been studied in resensitization to RAI therapy which will be discussed later in this chapter. It is also currently being studied in multiple clinical trials in combination with MEK inhibitors.
Sequential Administration
There is little data regarding sequential administration of TKIs in patients with progressive DTC and how these patients respond compared to treatment-naïve patients. In a small retrospective review, patients with progressive DTC on sorafenib were then treated with sunitinib. In one patient refractory to sorafenib, there was a 38% tumor reduction on second-line sunitinib [92]. In another trial comparing response to lenvatinib in treatment-naïve patients versus patients treated with prior VEGFR kinase inhibitors, the response rate in the treatment-naïve group was 54% compared to 41% in the previously treated group, suggesting continued activity of lenvatinib despite prior treatment [71]. In another trial evaluating the role of vemurafenib in BRAF-mutated DTC in pretreated patients and treatment-naïve patients, the partial response rate was also lower in pretreated patients (26%) compared to treatment-naïve patients (35%) [86].
Another retrospective review evaluated the efficacy of salvage therapy in patients with advanced DTC who failed first-line sorafenib treatment [93]. Salvage therapy included treatment with sunitinib, pazopanib, cabozantinib, lenvatinib, or vemurafenib in 17 patients who had previously failed sorafenib. Partial response rates were 41% in the salvage group compared to 13% in the sorafenib-only group. Median overall survival rates were also significantly longer in the salvage group (58 months) compared to the sorafenib-only group (28 months).
Given the mixed treatment responses in pretreated patients compared to treatment-naïve patients, more studies are required to evaluate the optimal sequencing of TKI use in progressive DTC.
Adverse Effects
Commonly seen adverse events specific to each TKI have been noted above. Certain adverse events that pertain to the drug class as a whole will be discussed below.
Hypertension. This is the most common adverse effect associated with TKIs with angiogenic effects. While its severity is dose related, certain studies have shown that it is a marker of efficacy as blood pressure over 140/90 was associated with improved survival [94]. According to the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee’s guidelines for management of TKI-induced hypertension, it is recommended that the goal blood pressure should be less than 140/90 prior to and during treatment with TKIs [95]. The choice of antihypertensive agent should be individualized. Patients should monitor their blood pressure daily and doctors weekly until goal blood pressure is achieved. Stopping the TKI or reducing the dose may be needed if blood pressure goals are not met.
QT Interval Prolongation on EKG. A baseline EKG and electrolyte panel should be performed in all patients being considered for a TKI. While all TKIs can cause QT prolongation, vandetanib carries a “black box” warning to QT interval prolongation, torsades de pointes, and sudden death. It is thus contraindicated with anyone with a history of or at high risk for those. Electrocardiograms should be performed at baseline, after 2–4 weeks, and every 3 months thereafter. Careful consideration to initiation of other drugs that may prolong QT interval should also be made.
Congestive Heart Failure. Although infrequent, TKIs have been associated with myocyte toxicity and both systolic and diastolic heart failure. Hypertension can also exacerbate this failure. Baseline evaluation with an echocardiogram should be considered, and periodic monitoring thereafter depends on patient symptomatology and clinician judgment. Standard heart failure therapy should be initiated if indicated. Discontinuation or interruption of the TKI should be considered if medical treatment fails to control the symptoms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree