Figure 20.1
Radiofrequency ablation (RFA). (a) Probe being advanced into the lesion, usually under ultrasound guidance. (b) Deploying the tines to the just beyond the edges of the lesion. (c) Zone of ablation extending beyond the tumor
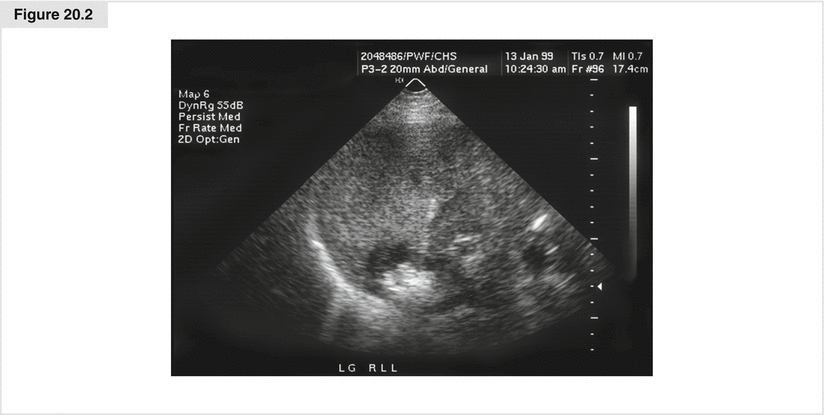
Figure 20.2
Image obtained under ultrasound during radiofrequency ablation. Note the needle advanced into the tumor in the posterior portion of the liver, with the white portion being the area within the tumor that has reached >100 °C
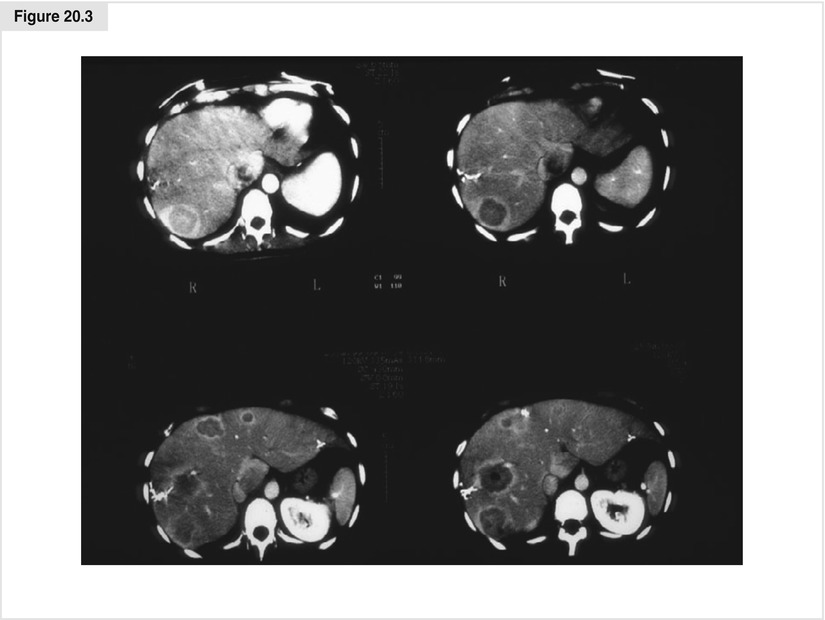
Figure 20.3
CT images of necrotic avascular tumors after RFA
20.3 Irreversible Electroporation
Irreversible electroporation (IRE) is a new technology that uses very high voltage (1500 V/m2) at very low direct current (nanoamperes) passing through tissue between the positive and negative electrodes for multiple brief pulses (nanoseconds). The flow of electricity creates irreparable holes in cell membranes, inducing apoptosis. This technique does not produce heat or destroy stromal framework, so it is desirable for use near intrahepatic blood vessels and bile ducts, where significant heat generation would produce collateral damage. Normal bile duct and endothelial cells repopulate along the remaining stromal framework. We employ IRE (NanoKnife®, AngioDynamics, Latham, NY, USA) when RFA would be too close to hilar structures to avoid damage to main bile ducts, usually for tumors of 3 cm or less. Two to four electrodes are placed into or near the tumor to create an electric current field of sufficient size to ablate the tumor (Fig. 20.4a).
Ultrasound or CT guidance is used to place the 18-gauge needles (Fig. 20.4b). It is important to place the electrodes parallel to each other in multiple planes to produce the desired current flow and maintain uniform tissue impedance. Placing needles 2.5 cm apart appears to be optimum spacing (Fig. 20.4c). It is also important to place the electrodes parallel to major vascular structures so as not to traverse the lumens with the needles, which will increase the risk of postprocedure thrombosis.
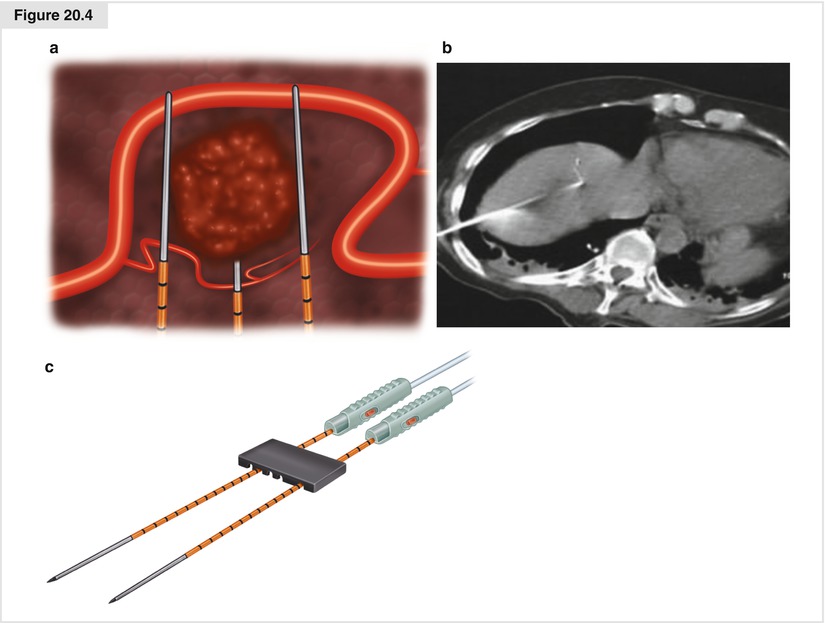

Figure 20.4
(a) Irreversible electroporation (IRE) electric field generated around the tumor. (b) Initial needle placement seen on CT scan for percutaneous technique. (c) Needle spacing
20.4 Tumor Enucleation
Often midgut NETs metastatic to the liver are surrounded by a pseudocapsule, and these tumors are very dense. They can be enucleated (Figs. 20.5 and 20.6) by crushing the hepatic parenchyma near the tumor with a hemostat, clipping the feeding structures with fine hemoclips, and tying the larger vessels prior to division. Alternatively, an energy resective device such as the LigaSure™ (Medtronic–Covidien, Minneapolis, MA, USA) can be used. Enucleation preserves the greatest amount of normal intervening liver and is useful for multiple or single superficial tumors of the liver. It also can be done laparoscopically. Despite positive margins of resection, recurrence rates remain low using this technique.
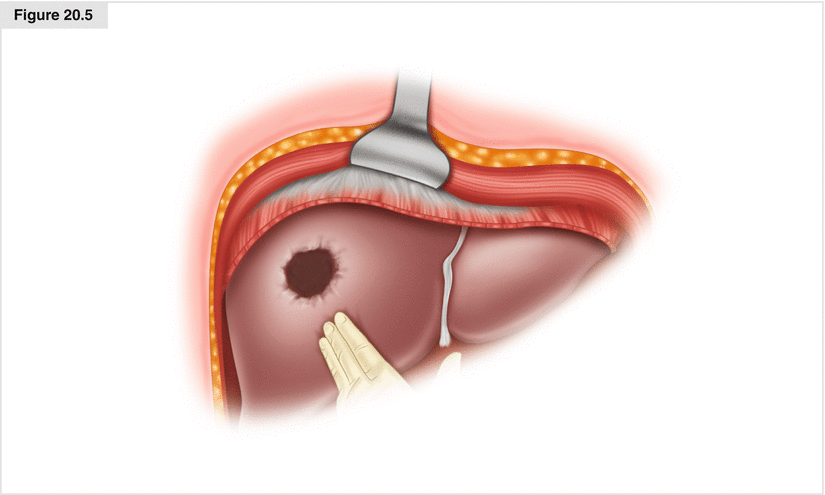

Figure 20.5
Neuroendocrine tumor (NET) after enucleation

Figure 20.6
Enucleated NET specimen
20.5 Segmental and Lobar Resections
More formal segmental or lobar resections are performed along the Couinaud resection lines (Fig. 20.7a) following the venous system. Ultrasound is used to note the location of the veins within the liver. The larger trunks are not amenable to sealing and dividing with the energy devices currently available (LigaSure™; Harmonic® scalpel, Ethicon Endo-Surgery, Cincinnati, OH; Aquamantys®, Medtronic, Minneapolis, MN), but the smaller branches entering the segments can usually be adequately sealed with these devices. The crush and clip or tie technique and/or an energy device is used to divide the hepatic parenchyma. Liver compression sutures of 2-0 chromic on a blunt needle are used as an adjunct for hemostasis along resection lines (Fig. 20.7b). Intraoperative ultrasound should be employed to map out the larger vascular structures; these can be divided with the endoscopic vascular stapler inserted directly into the hepatic parenchyma (Fig. 20.7c), or by conventional suture ligature. This technique is best applied to superficial lesions. Despite tumor cells at the margin, recurrence rates are generally low.
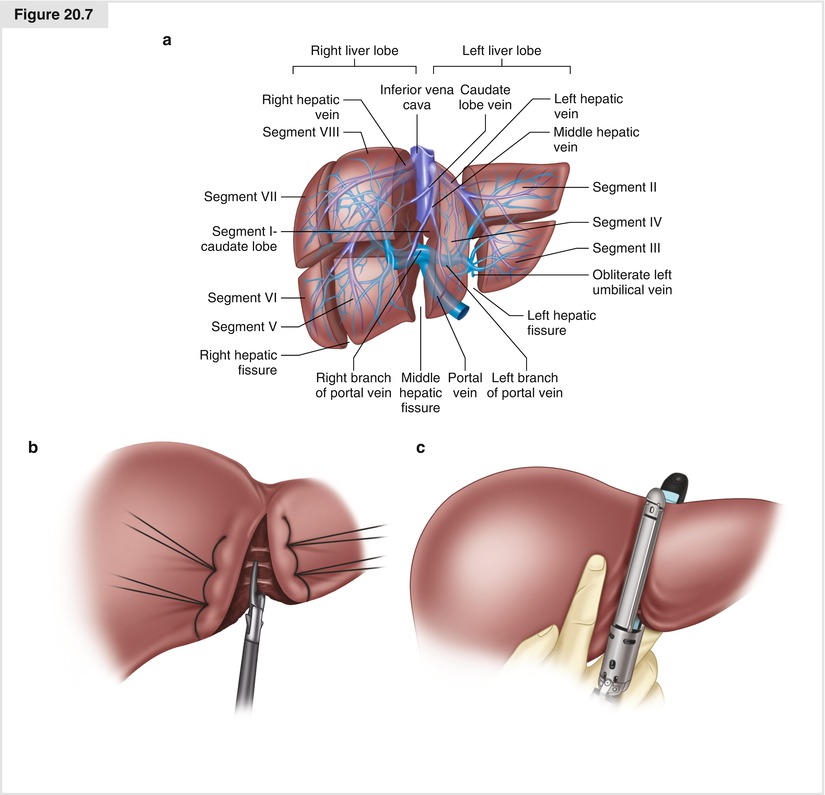

Figure 20.7




(a) The Couinaud segmentation of the liver. (b) Liver compression sutures and parenchymal division with an energy device. (c) Use of an endovascular stapler in a left lateral segmentectomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree