(T) Primary Tumor | Adapted from 7th edition AJCC Staging Forms. |
TNM | Definitions |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
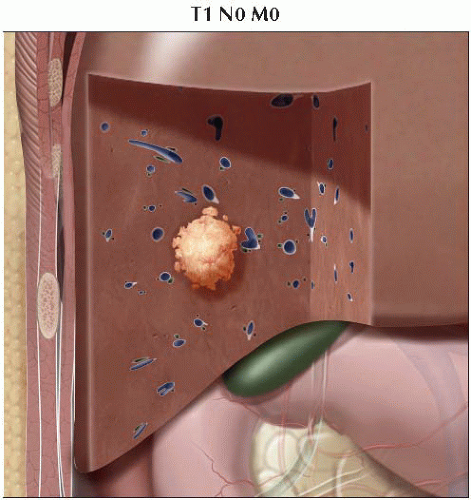
T1 | Solitary tumor without vascular invasion |
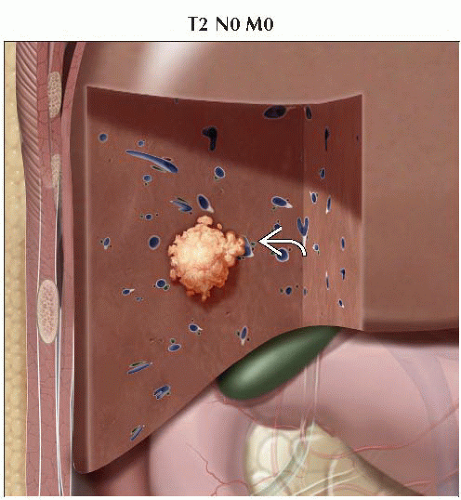
T2 | Solitary tumor with vascular invasion or multiple tumors, none > 5 cm |
T3a | Multiple tumors > 5 cm |
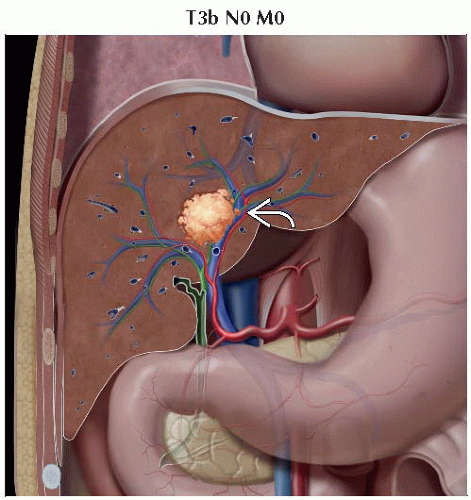
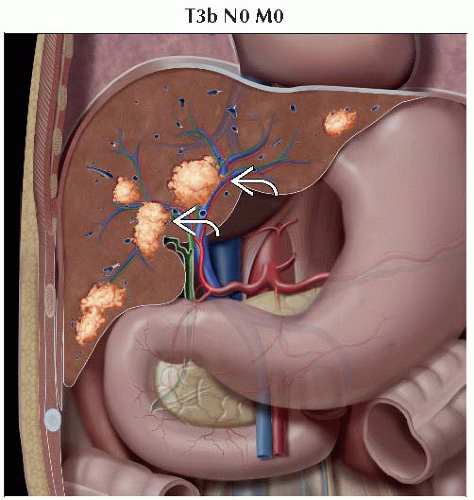
T3b | Single tumor or multiple tumors of any size involving a major branch of the portal vein or hepatic vein |
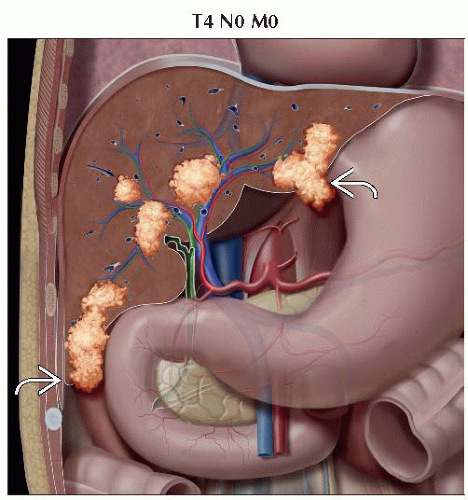
T4 | Tumor(s) with direct invasion of adjacent organs other than the gallbladder or with perforation of visceral peritoneum |
(N) Regional Lymph Nodes | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
(M) Distant Metastasis | |
M0 | No distant metastasis |
M1 | Distant metastasis |
(G) Histologic Grade | |
G1 | Well differentiated |
G2 | Moderately differentiated |
G3 | Poorly differentiated |
G4 | Undifferentiated |
AJCC Stages/Prognostic Groups | Adapted from 7th edition AJCC Staging Forms. | ||
Stage | T | N | M |
I | T1 | N0 | M0 |
II | T2 | N0 | M0 |
IIIA | T3a | N0 | M0 |
IIIB | T3b | N0 | M0 |
IIIC | T4 | N0 | M0 |
IVA | Any T | N1 | M0 |
IVB | Any T | Any N | M1 |
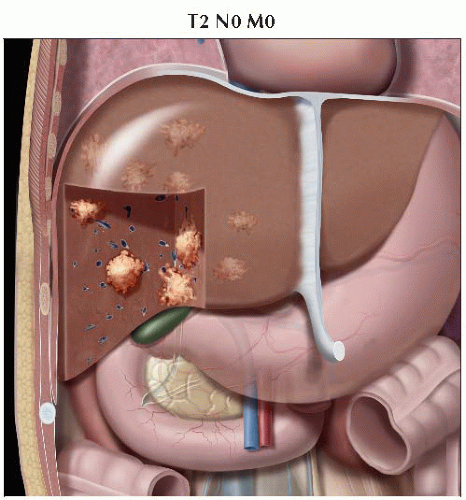 Graphic shows multiple tumors throughout the liver, all of which are smaller than 5 cm. No vascular invasion is seen, consistent with T2 disease. |
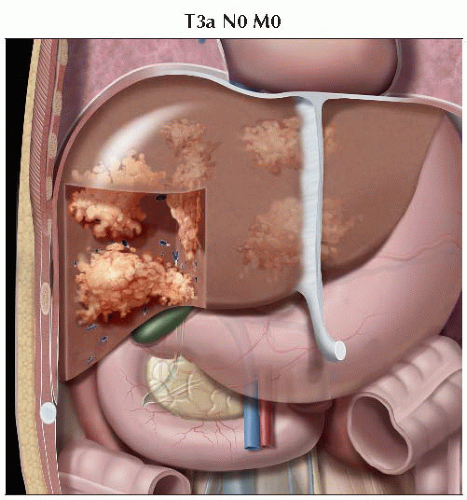 Graphic shows multiple tumors throughout the liver measuring more than 5 cm, consistent with T3a disease. |
Most common primary hepatic malignant tumor
Synonymous with hepatoma
Hepatocellular carcinoma (HCC)
Usually occurs in setting of cirrhosis
Poor prognosis
Fibrolamellar carcinoma
Relatively rare variant of HCC
Does not occur in setting of cirrhosis
Better prognosis than conventional HCC
Comments
Carcinogenesis of HCC in cirrhosis
Commonly described as multistep evolution of cirrhotic nodules
International Working Party describes 2 types of cirrhotic nodules
Regenerative nodules
Localized proliferation of hepatocytes and supporting stroma
Response to local hepatocellular damage
Hyperplasia secondary to deficient portal venous perfusion → early arterial neovascularity
Dysplastic nodules
Hepatocytes that undergo abnormal growth due to genetic alteration
Histologic precursor to HCC
Small HCCs (< 2 cm) are often histologically indistinguishable from dysplastic nodules
↑ arterial neovascularity compared to regenerative nodules
Risk factors
HCC usually occurs in setting of cirrhosis (90%)
Common causes of cirrhosis include
Viral hepatitis (individuals with chronic hepatitis are at 20x greater risk of developing HCC)
Hepatitis C virus (accounts for 55% of cirrhosis)
Hepatitis B virus (accounts for 16% of cirrhosis)
Alcoholism
Carcinogens
Aflatoxins: Particular populations found in sub-Saharan Africa and China have a mutated hepatic enzyme that fails to deactivate aflatoxin
Activated aflatoxin interacts with specific sites within p53 gene contributing to high incidence of HCC
Thorotrast (particularly in development of cholangiocarcinoma)
Androgens
Hemosiderosis (from repeated blood transfusions)
Metabolic disorders
α-1-antitrypsin deficiency
Hemochromatosis
Wilson disease
Tyrosinosis
Protective factors
Universal vaccination of children against HBV in endemic areas (began in Taiwan in 1984 and has markedly decreased HBV infection rate)
Number of cases in USA per year
35,557 (2011)
Sex predilection
M:F = 8:1 (in countries with high incidence); 2-3:1 (in countries with low incidence)
Age of onset
Median age of diagnosis is 63 years
3rd leading cause of death from cancer worldwide
Accounts for 250,000 deaths worldwide each year
Incidence of HCC in developing nations is more than double that of developed countries
Incidence of HCC is highest in Asia and Africa due to high prevalence of hepatitis B and C
Frequency in United States
Incidence in USA has more than doubled in last 20 years from 2.6 to 7.5 per 100,000 population
There is an increased incidence in USA amongst Asian/Pacific Islander men (22:100,000)
75% of cases occur in men compared to women (11.6 vs. 3.9 per 100,000)
Racial distribution
Caucasian (48%)
Hispanic (15%)
African American (14%)
Other, predominantly Asian (24%)
Common translocations
Aflatoxin acts on guanosine base in codon 249 of p53 tumor suppressor gene leading to a G to T transversion
This is often identified in HCC found in regions of sub-Saharan Africa and China, contributing to high incidence in these regions
HBV
Viral DNA integrates into host’s genomic DNA in tumor cells
HBV X protein acts as a transactivator of cellular and viral promoters
Disrupts normal cell growth
Also binds to p53 tumor suppressor gene
Cirrhosis
Repeated cycles of cell death and regeneration lead to accumulation of mutations
Important component of the pathogenesis of HCV-and HBV-associated liver cancer
Fibrolamellar carcinoma
Variant of hepatocellular carcinoma
Relatively rare neoplasm with better prognosis than conventional HCC
Occurs most commonly in absence of cirrhosis
Affects younger age group with peak incidence at 24.8 ± 8 years
Higher incidence among Caucasians
No gender predilection
Cholangiocarcinoma
15-20% of primary liver tumors
2nd most common primary liver tumor (80% extrahepatic subtype)
30-50% have nodal spread at presentation
10-20% have distant metastasis at presentation
Similar risk factors as hepatocellular, but also
Primary sclerosing cholangitis, tobacco smoke, chronic ulcerative colitis in Western countries, and endobiliary infections in Asia (liver flukes)
Incidence ↑ in USA (0.7 per 100,000)
Average age of diagnosis is 73 years
Metastatic disease
Secondary liver cancer is ˜ 20x more common than primary
Most commonly secondary to colorectal cancer
5-year survival < 5% with hepatic failure most common cause of death
Surgical resection may achieve 5-year survival of 37-58% but only 20-25% are eligible
Most metastatic cancers are adenocarcinomas that quickly outgrow blood supply and thus have a central necrotic region on imaging
Classic macroscopic classification proposed by Eggle in 1901 is still used today
Nodular
Smaller and more distinct than massive lesions
Sharper margins
Massive: 2 dominant forms
Composed of confluent small tumors
1 large lesion occupying almost entire liver
Diffuse
Multiple infiltrating lesions occupying large part of liver
H&E
Edmondson grading system widely used to grade histology of HCC
Grade I
Tumor cells similar in size to normal hepatocytes
Arranged in relatively thin trabeculae
Acini containing bile are rare
Grade II
Cells larger than normal hepatocytes
Hyperchromatic nuclei occupy greater proportion of cells
Thicker trabeculae
Acini containing bile are common
Grade III
Hepatocytes with large nuclei that occupy > 50% of cytoplasm
Trabeculae still dominant although isolated cells may be present
Giant and bizarre cells common
Bile is rarely present
Grade IV
Cells contain nuclei that occupy most of cytoplasm
Predominantly solid areas with little or no bile
Intravascular and intrasinusoidal growth common
Special stains
Reticulin stain commonly used to visualize reticular fibers
Hep-Par 1
Commonly used immunostain for suspected HCC
Highly sensitive and specific for hepatocytic differentiation
Local spread
3 distinct intrahepatic forms have been commonly described
Solitary massive tumor
Multiple nodules scattered throughout liver
Diffuse infiltration of liver
Vascular invasion commonly seen
Hepatic vein invasion may lead to Budd-Chiari syndrome
Portal venous invasion
Lymphatic extension
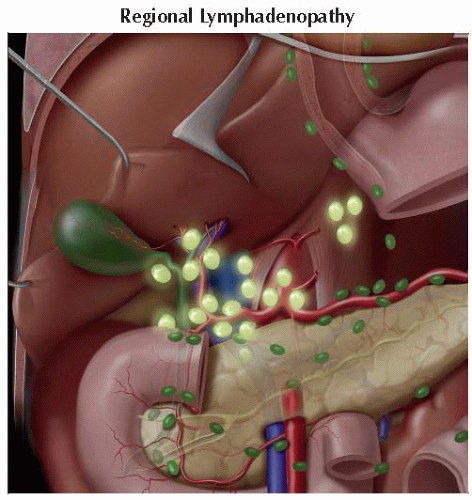
Regional lymphadenopathy implies N1 disease by TNM criteria
Regional nodal involvement (in order of prevalence)
Periceliac
Portohepatic
Paraaortic
Portocaval
Peripancreatic
Aortocaval
Retrocaval
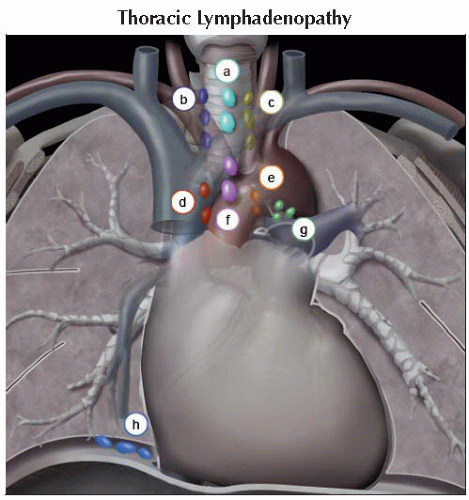
Distant lymphadenopathy (in order of prevalence)
Mediastinal
Cardiophrenic
Mesenteric
Internal mammary
Perirectal
Retrocrural
Iliac
Paraspinal
Hematogenous spread
Hematogenous spread is relatively uncommon despite obvious vascular invasion
Metastatic sites
Distant metastases (in order of prevalence)
Lungs
Musculoskeletal sites
Adrenal gland
Peritoneum &/or omentum
Cirrhosis alters normal liver morphology with variable degree of
Fibrosis
Scarring
Nodular regeneration
Altered hepatic perfusion
Portal hypertension
Portal venous occlusion ± reversal of flow
Regenerative nodules
Ultrasound
Plays little role in detection of discrete liver nodules
Liver margins may demonstrate nodular contour in setting of macronodular cirrhosis
CT
Nodules poorly visualized on NECT
Enhancement similar to background parenchyma on CECT
Siderotic nodules may be occasionally seen as hyperdense on NECT
MR
T1WI
Nonsiderotic nodules can occasionally be detected as slightly hyperintense
Siderotic nodules well visualized on gradient-echo images, but rarely seen on spin-echo images
T2WI
Nonsiderotic nodules rarely seen
Siderotic nodules well visualized as discrete hypointense foci
T1 C+
Nonsiderotic nodules poorly visualized (may very rarely demonstrate arterial phase enhancement)
Siderotic nodules commonly seen as hypointense foci
Dysplastic nodules
Ultrasound
Plays little role in detection of liver nodules
CT
Nodules may occasionally be seen as hyperdense on NECT
Generally isodense to liver on CECT
MR
T1WI
Large nodules may be homogeneously hyperintense
T2WI
Large nodules may be homogeneously hypointense
T1 C+
Enhancement rare
Mimics HCC when seen
Hepatocellular carcinoma
Ultrasound
Usual modality of choice for screening of HCC in cirrhotic patient
Most affordable imaging modality
No ionizing radiation
Echogenicity of HCC highly variable
Small lesions (< 5 cm) are usually hypoechoic
Thin hypoechoic halo corresponding to fibrous capsule commonly seen
Larger lesions (> 5 cm) are generally mixed echogenicity
Hyperechoic areas can be seen in setting of intratumoral fat
Hypoechoic regions commonly seen in setting of necrosis
Color Doppler
Neovascularity and arteriovenous shunting may be seen
High-velocity waveforms characteristic, albeit nonspecific
Power Doppler signal variable; cannot be used to reliably distinguish HCC from metastatic disease
CT
NECT
Visualization generally limited without IV contrast
Lesions are usually hypodense if detected
Patchy fat attenuation may be seen in lesions with intratumoral fat
Fluid attenuation may be seen with tumoral necrosis
CECT
Arterial phase
Avid homogeneous enhancement in small lesions
Heterogeneous enhancement in larger lesions
Transient hepatic attenuation difference may be seen as wedge-shaped region of ↑ perfusion from local portal vein occlusion
Some advocate both early and late arterial phases to overcome differences in blood flow kinetics and tumor characteristics
Portal venous phase
Small lesions usually not detectable due to washout
Larger lesions may retain variable degree of enhancement
Delayed phase
Both small and large lesions generally not well visualized
Hepatic artery catheter CT (“coned-beam CT”)
MR
Most sensitive and specific imaging modality for detection of HCC
Considered gold standard for characterization of liver nodules in setting of cirrhosis
T1WI
Variable signal, depending on degree of fatty metaplasia, fibrosis, and necrosis
Generally iso- to hypointense
Rarely hyperintense in presence of fat, copper, or glycoproteins
T2WI
Variable signal although generally hyperintense
“Nodule within nodule” occasionally seen (small T2 hyperintense focus within uniformly T2 hypointense dysplastic nodule)
T1 C+
Small lesions (< 2 cm) generally show rapid arterial enhancement with rapid washout in portal venous and delayed phases
Large lesions (> 2 cm) demonstrate heterogeneous nodular enhancement during both arterial and later phases
Diffusion-weighted imaging (DWI)
Evolving technology used in conjunction with other imaging sequences
Useful tool for lesion detection
DWI presently limited for lesion characterization; good for follow-up post TACE and radioembolization
Arteriography
Enlarged arterial feeders
Coarse neovascularity
Arterioportal shunts
“Threads and streaks”
Linear parallel vascular channels coursing along portal venous radicles
Seen in setting of portal venous involvement
3D C-arm cone-beam CT
Hepatic arteriography with concomitant 3D cross-sectional imaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree