Lenalidomide was approved by the US Food and Drug Administration (FDA) for treatment of transfusion-dependent lower-risk myelodysplastic syndrome patients with deletion (del) (5q) alone or with additional karyotype abnormalities. The approval was based on high rates of prolonged transfusion independence and complete cytogenetic response in this subset. In lower-risk non-del(5q) patients, meaningful erythroid responses also were reported with a low frequency of cytogenetic improvement, although inferior to that observed in the del(5q) patients. There is now a better understanding of the mechanism of the karyotype-dependent drug action, explaining the disparate response rates and frequency of myelosuppression. In del(5q) patients, lenalidomide suppresses the clone by inhibiting the nuclear sequestration of the haplodeficient cell cycle regulatory protein cdc25c, thereby promoting selective G2 arrest and apoptosis. In non-del(5q) patients, lenalidomide enhances erythropoietin receptor signaling. Future directions include use of biologic and molecular markers as predictive tools to select patients and use of combination strategies to overcome resistance to lenalidomide in del(5q) patients or enhance erythropoeisis in non-del 5 patients.
Myelodysplastic syndromes (MDS) are a spectrum of hematopoietic stem cell malignancies characterized pathologically by the presence of cytologic dysplasia and clinically by bone marrow failure with persistent and progressive variant cytopenias. More than 90% of patients diagnosed with MDS will have anemia during their disease course, and 30% to 50% of patients will be transfusion-dependent, more so in higher risk patients. Red blood cell (RBC) transfusion dependency is an independent adverse prognostic factor in MDS.
Anemia is predominantly the result of ineffective erythropoeisis. Accelerated apoptosis is the hallmark of early disease, while up-regulation of survival signals, proliferation, and clonal evolution are features of higher-risk disease. Impaired clonogenic growth of primitive erythroid progenitors and impaired erythropoietin receptor signaling are major causes of anemia. Inflammatory cytokines amplify apoptosis of hematopoietic progenitors and the inherent defective erythroid maturation. Immunologic derangements can contribute to anemia also.
In the past, options for treating anemia were limited to the use of erythroid stimulating factors (ESAs) and RBC transfusions. Unfortunately, only 20% to 40% of patients will respond to ESAs alone or ESA combined with granulocyte colony-stimulating factor (G-CSF). In the past 5 years, three drugs were approved for the treatment of MDS. Lenalidomide, a second-generation, immunomodulatory drug (IMiD), was approved by the US Food and Drug Administration (FDA) in December 2005 for treatment of transfusion-dependent anemia in patients with lower–risk MDS with interstitial deletion of a segment of the long arm of chromosome 5 (del[5q]). This article summarizes the relevant data that led to the FDA approval of lenalidomide in patients with del(5q), discusses the role of lenalidomide in the treatment of anemia in non-del(5q) patients, highlights some of the new insights into the mechanism of action and resistance, and finally discusses current and future strategies of using lenalidomide in the treatment of MDS.
The rationale for lenalidomide in MDS
Thalidomide paved the road for the use of lenalidomide for the treatment of MDS. The story of thalidomide’s evolution into an established anticancer agent is well known, including both its triumph and tragedy.
IMiDs exert a variety of biologic effects, including their action to modify ligand-induced receptor signals that include inflammatory cytokine generation, antiangiogenic effects, immune and cell adhesion response, and direct antiproliferative effects. All those effects make the use of IMiDs appealing for treating MDS patients.
In MDS, stromal abnormalities and immune changes lead to production of inflammatory and hematopoietic inhibitory cytokines, such as tumor necrosis factor (TNF)-α. IMiDs exert different cytokine modulation effects based on the type of cell and stimulus. IMiDs decrease TNF-α production from monocytes after lipopolysaccharide or microbial stimulation, while they potentiate TNF-α production through T cell costimulation to enhance immune response. Lenalidomide is a 100- to 50,000-fold more potent inhibitor of monocyte TNF-α production than thalidomide.
Angiogenesis is thought to contribute to the progression of MDS to higher-risk disease. IMiDs’ angiogenic properties derive in part from suppression of vascular endothelial growth factor (VEGF) production and VEGF cellular response. IMiDs decrease microvessel density and inhibit new vessel growth in rat models, and this has been evidenced from correlative studies in clinical trials.
Different immune alterations have been described in MDS. In lymphoid cells, lenalidomide facilitates and potentiates T cell costimulation in response to antigen activation to enhance CD8 + and NK cell-mediated cytotoxicity. It also restores the balance between CD-4 + T helper Th1 and Th2 cells.
Thalidomide was the first IMiD tested in MDS. Overall, hematologic improvement rates approximated 20% and mainly were restricted to the erythroid lineage. The erythroid responses, however, were robust and often longstanding. The toxicity of long-term use of thalidomide, namely neuropathy, precluded further development, and lenalidomide, based on its more favorable activity/toxicity profile, emerged as the next IMiD for clinical development.
Efficacy of lenalidomide in MDS
Lenalidomide’s activity and safety in the treatment of MDS patients was established in three consecutive clinical studies.
The MDS-001 study was the exploratory single-institution phase 1/2 study. This trial enrolled 43 patients with MDS who had either symptomatic anemia or were transfusion-dependent (defined as need for 4 U of RBC within 8 weeks before enrollment). All patients had failed treatment with recombinant erythropoietin (EPO) (77%) or had low probability of response to an ESA based an endogenous serum EPO level and heavy transfusion burden (23%). The study excluded patients with neutropenia less than 500/mm 3 or platelets less than 10,000 mm 3 . Most patients (77%) had refractory anemia (RA) or RA with ringed sideroblasts (RARS), and 88% were low-to-intermediate-1 (int-1) risk scores according to the International Prognostic Scoring System (IPSS). Two thirds of the patients were transfusion-dependent, and 30% failed treatment with thalidomide. An abnormal karyotype was present in 43% of patients, and 12 patients had a del(5q) abnormality. The overall hematologic response rate was 56%. Twenty-one patients (49%) achieved a major erythroid response. Among transfusion-dependent patients, 63% achieved transfusion independence. The most important finding was the significant relation between cytogenetics and hematologic response, where 83% of patients with a del(5q) abnormality had an erythroid response, compared with 57% of those with a normal karyotype and 12% of those with other cytogenetic abnormalities ( P = .007). Among 20 patients with an abnormal karyotype, 11 patients achieved a cytogenetic response. Out of the 12 patients with del(5q) abnormality, 10 (83%) had a cytogenetic response, and 9 (75%) had a complete cytogenetic response.
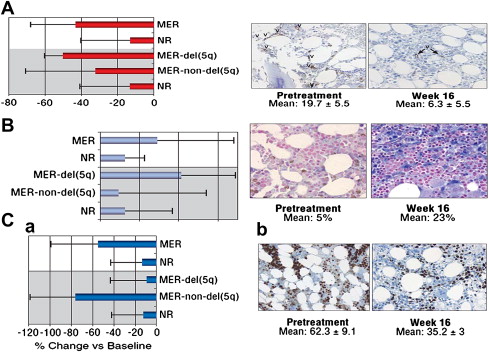
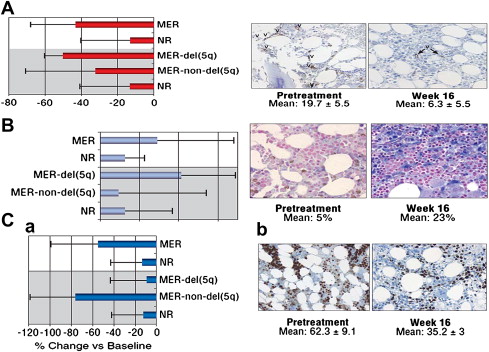
Correlative biologic studies confirmed much of the proposed rationale for the use of lenalidomide in MDS. Bone marrow concentrations of proapoptotic cytokines decreased significantly in responders to lenalidomide; erythroid maturation also increased with a significant reduction in microvessel density in responder patients, particularly in patients with del(5q) abnormality. The biomarker studies also supported a karyotype-specific mechanism of action, where reduction in microvessel density was greatest in patients with del(5q), while reduction in proliferative index was greater in non-del(5q) erythroid responders ( Fig. 1 ).
Efficacy of lenalidomide in MDS
Lenalidomide’s activity and safety in the treatment of MDS patients was established in three consecutive clinical studies.
The MDS-001 study was the exploratory single-institution phase 1/2 study. This trial enrolled 43 patients with MDS who had either symptomatic anemia or were transfusion-dependent (defined as need for 4 U of RBC within 8 weeks before enrollment). All patients had failed treatment with recombinant erythropoietin (EPO) (77%) or had low probability of response to an ESA based an endogenous serum EPO level and heavy transfusion burden (23%). The study excluded patients with neutropenia less than 500/mm 3 or platelets less than 10,000 mm 3 . Most patients (77%) had refractory anemia (RA) or RA with ringed sideroblasts (RARS), and 88% were low-to-intermediate-1 (int-1) risk scores according to the International Prognostic Scoring System (IPSS). Two thirds of the patients were transfusion-dependent, and 30% failed treatment with thalidomide. An abnormal karyotype was present in 43% of patients, and 12 patients had a del(5q) abnormality. The overall hematologic response rate was 56%. Twenty-one patients (49%) achieved a major erythroid response. Among transfusion-dependent patients, 63% achieved transfusion independence. The most important finding was the significant relation between cytogenetics and hematologic response, where 83% of patients with a del(5q) abnormality had an erythroid response, compared with 57% of those with a normal karyotype and 12% of those with other cytogenetic abnormalities ( P = .007). Among 20 patients with an abnormal karyotype, 11 patients achieved a cytogenetic response. Out of the 12 patients with del(5q) abnormality, 10 (83%) had a cytogenetic response, and 9 (75%) had a complete cytogenetic response.
Correlative biologic studies confirmed much of the proposed rationale for the use of lenalidomide in MDS. Bone marrow concentrations of proapoptotic cytokines decreased significantly in responders to lenalidomide; erythroid maturation also increased with a significant reduction in microvessel density in responder patients, particularly in patients with del(5q) abnormality. The biomarker studies also supported a karyotype-specific mechanism of action, where reduction in microvessel density was greatest in patients with del(5q), while reduction in proliferative index was greater in non-del(5q) erythroid responders ( Fig. 1 ).
Lenalidomide in lower-risk MDS with del(5q)
The MDS-003 study ( Table 1 ) was the pivotal study for the FDA approval of lenalidomide for lower-risk MDS patients with del(5q) abnormality. The study design and eligibility criteria were similar to the MDS-001 study; however, eligibility was limited to patients with a del(5q) abnormality. The study enrolled 148 patients. Treatment consisted of lenalidomide 10 mg daily for 21 days every 28-day cycle. Shortly after the study activation, the schedule was amended to a continuous schedule given the apparent faster time to response observed with this schedule in the MDS-001 study. Sixty-four percent of the patients had the French American British subtypes RA or RARS, and most were low or int-1 IPSS. Two thirds of the patients had isolated del(5q), but only 26% overall had the 5q syndrome. Most patients were treated previously with an ESA (73%) and were heavily transfusion-dependent (71%, 2 U or more).
| Number of patients | 148 |
| Median age | 71 years |
| Baseline characteristics | IPSS risk category—number (%) Low 55 (37) Intermediate—1 65 (44) Intermediate—2 or high 8 (5) Unclassified 20 (14) Karyotype—number (%) Isolated 5 out of every 110 (74) 5q + additional abn 37 (25) |
| Responses | Overall erythroid response 112 (76%) Transfusion independency (TI) 99 (67%) ≥50% transfusion reduction 13 (9%) TI frequency by karyotype complexity Isolated del(5q) 79 (72%) Del(5q) + 1 additional 12 (48%) Complex (>30) 8 (67%) Overall cytogenetic response 62/85 (73%) Complete response 38/85 (45%) Median Hgb increase 5.4 g/dL |
| Time to response | Median time to response 4.6 weeks Median duration of response 115 weeks |
| Hematological toxicity (number of patients [%]) | Grade 3 or 4 Neutropenia 81 (55) Thrombocytopenia 65 (44) Anemia 10 (7) |
The overall transfusion response rate was 76% (112 patients). Ninety-nine patients (67%) achieved transfusion independence, and 13 patients (9%) had more than a 50% reduction in transfusion. The median time to response was short (4.6 weeks), and the median rise in Hgb compared with baseline was 5.4 g/dL. The duration of response was durable, lasting more than 2 years, even longer for patients with isolated del(5q). Forty-nine patients (77%) with isolated 5q achieved a cytogenetic response after 24 weeks of treatment, including 45% with a complete cytogenetic response. In patients with del(5q) and one additional abnormality, 67% had a cytogenetic response and 40% having a complete response. The cytogenetic response rate was 50% in patients with a complex karyotype. In del(5q) MDS patients, development of a treatment-induced cytopenia in the first 8 weeks on lenalidomide treatment (at least 50% decrease in platelets or at least 75% decrease in neutrophil count) was associated with a higher rate of hematologic response and cytogenetic response, indicating that early cytopenias may be a surrogate marker for suppression of the del(5q) clone.
Among the patients with del(5q) treated on MDS-001 and MDS-003, cytogenetic response strongly correlated with extended survival (hazard ratio [HR], 5.295; P <.001). Kaplan-Meier estimates of overall survival from the start of lenalidomide treatment showed that cytogenetic responders had a significant survival advantage compared with nonresponders (NR) (median, not reached vs 28 months; P <.0001). The 10-year survival estimate for cytogenetic responders was 78% compared with 4% in the NR cohort. The 10-year estimated risk for leukemia progression was 15% in responding patients compared with 67% in the NR group ( P = .010).
In a small cohort of European patients (n = 22) enrolled on MDS-003 progression to acute mycloid leukemia (AML) was reported in 8 patients (two patients with del(5q) syndrome, four with refractory cytopemia with multi lineage dysplasia, 2 with refractory anemia with excess blasts). Progression to AML was accompanied by development of complex karyotype clonal evolution in seven of those patients. It is not clear if lenalidomide increases chromosomal instability or if this reflects the natural course of the disease. More recent data suggest that the median overall survival for patients with untreated del(5q) may be shorter than what originally was estimated. The median overall survival was 3 years in a prospective analysis of German patients with del(5q) before introduction of lenalidomide.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree