Hormone
Productiona
Effect on appetite
CCK
I cells of duodenum and jejunum
↓
GLP1
L cells of distal intestine and colon
↓
Oxyntomodulin
L cells of distal intestine
↓
PYY3–36
L cells of distal intestine
↓
Amylin
β cells of pancreas
↓
PP
Pancreatic islet cells
↓
Ghrelin
Epithelial cells of gastric fundus
↑b
Cholecystokinin
Cholecystokinin (CCK) was the first GI peptide shown to have an anoretic effect and is considered to be the prototypical satiety hormone (Wren and Bloom 2007; Liebling et al. 1975; Ahima and Antwi 2008; Cummings and Overduin 2007). Intestinal CCK is mainly produced by the I cells of the duodenal and jejunal mucosa in response to intra-luminal protein and fat (Cummings and Overduin 2007). Once released, CCK acts locally within the GI tract on vagal CCK-A receptors in order to induce satiety, delay gastric emptying, stimulate pancreatic enzyme secretion, and produce gallbladder wall contractions. The administration of CCK-A receptor antagonists to patients has been shown to increase hunger, meal size, and caloric intake (Berlinger et al. 2001); thus, CCK has been studied as a potential therapeutic target in the bariatric population. However, the very short-lived anoretic effects of CCK make its role in long-term metabolic homeostasis uncertain. In fact, trials investigating the administration of CCK and CCK-A receptor agonists for sustained weight loss have been unsuccessful to date (Cummings and Overduin 2007; West et al. 1984).
Glucagon-like Peptide-1
Glucagon-like peptide-1 (GLP1) is widely produced in the distal small intestine and colon by mucosal L cell in response to intra-luminal fat and carbohydrates (Cummings and Overduin 2007). The release of GLP1 results in the activation of both peripheral and central GLP1 receptors, which are found in the vagus nerve, proximal GI tract, pancreas, brainstem, and hypothalamus. Thus, the effects of GLP1 are both the result of vagal stimulation and direct activation of central GLP1 receptors after release into the bloodstream. The role of GLP1 appears to be decreasing food intake by inhibiting proximal intestinal motility, accentuating insulin release, inhibiting glucagon secretion, and stimulating the growth of pancreatic β cells (Cummings and Overduin 2007).
Oxyntomodulin
Oxyntomodulin, like GLP1, is a proglucagon-derived peptide produced by mucosal L cells in the distal intestine in response to ingested nutrients (Cummings and Overduin 2007). The exact signaling mechanisms for oxyntomodulin have not been entirely elucidated. However, given its similar origin and distribution to GLP1, it is believed that GLP1 receptors are involved even though oxyntomodulin and GLP1 target different regions in the brain (Cummings and Overduin 2007). Oxyntomodulin functions to reduce food intake, increase energy expenditure, and decrease body weight (Baggio et al. 2004). Repeated sub cutaneous injections of oxyntomodulin in patients over a 4-week period were shown to decrease the body weight by 0.5 kg/week and reduce food intake by 25 % (Wynne et al. 2005). Therefore, it is hoped that further study into oxyntomodulin could potentially produce therapeutic options for the bariatric patient.
Peptide YY (PYY)3–36
PYY3–36 is the bioactive form of PYY, which is co-secreted from L cells in the mucosa of the distal intestine with GLP1 and oxyntomodulin (Cummings and Overduin 2007). Like its co-secreted peptides, PYY3–36 is secreted in response to nutrients in the distal small bowel. Its receptors and targets are not clearly delineated, but PYY3–36 appears to induce satiety by delaying gastric emptying. PYY3–36 infusion has been shown to decrease food intake by up to 30 % in obese individuals and up to 31 % in lean individuals for up to 2 h after peripheral administration (Batterham et al. 2003a, b). This same study showed that participants had a lower 24-h caloric intake after PYY3–36 infusion in comparison to patients receiving a placebo infusion. However, other studies have shown that PYY3–36 infusion has variable effects on animal and human subjects depending on the dose, route, and timing of infusion (Cummings and Overduin 2007). Therefore, more research is certainly needed if PYY3–36 is to be considered as a potential therapeutic target.
Amylin
Amylin is co-secreted with insulin from pancreatic β cells after the ingestion of nutrients. It acts to inhibit gastric emptying, gastric acid and pancreatic enzyme production, and glucagon secretion (Cummings and Overduin 2007). Currently, the amylin analogue, pramlintide, is available as a treatment for diabetes but has also been shown to have the added benefit of inducing mild weight loss in patients (Hollander et al. 2004).
Pancreatic Polypeptide
Pancreatic polypeptide (PP) is secreted from specialized pancreatic islet cells in response to an ingested meal (Cummings and Overduin 2007). It acts both centrally and peripherally to adjust pancreatic exocrine functions, gastric acid secretion, and the motility of the GI tract. Studies have been conflicting about its role in metabolic homeostasis (Cummings and Overduin 2007), even though one study showed that the peripheral administration of PP to humans resulted in a decrease in appetite and food intake (Batterham et al. 2003a, b).
Ghrelin
Kojima et al. (1999) first described the structure and function of a hormone found in high concentrations in the rat stomach, which they termed “ghrelin”—a highly conserved homologous form of the hormone was also found in the human stomach. Ghrelin is a 28 amino acid peptide that is cleaved from its precursor, preproghrelin. Ghrelin acts upon growth hormone secretagogue receptors (GHS-R) in the hypothalamus. The highest concentration of ghrelin is within the gastric fundus, where nearly three quarters of ghrelin is produced by fundal epithelial cells (Wren and Bloom 2007). Additional sites of ghrelin production include the mucosa of the GI tract (most prominently in the duodenum), pancreas, ovaries, adrenal cortex, and the brain; although, production in these other areas is significantly less than that seen in the gastric fundus. The signaling mechanisms for ghrelin involve both vagally mediated pathways and the direct activation of receptors in the hypothalamus after release into the bloodstream (Wren and Bloom 2007; Kojima et al. 1999).
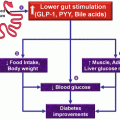
Ghrelin plays a unique role in regard to the initiation of meals and the maintenance of metabolic homeostasis as it is the only known orexigenic, or appetite-stimulating, hormone. After release, ghrelin induces hunger, increases GI motility, and suppresses insulin production (Cummings and Overduin 2007). Ghrelin plays a significant role in the initiation of meals and mealtime hunger as plasma levels of the hormone rise significantly before meals and have been shown to decrease in response to the ingestion of nutrients, most especially carbohydrates (Cummings et al. 2002; Cummings and Overduin 2007). However, apart from these short-acting effects, there is strong evidence to suggest that ghrelin is involved in long-term metabolic homeostasis. For example, plasma ghrelin levels negatively correlate with patient’s body mass index (BMI), overweight individuals have been shown to experience an increase in circulating plasma ghrelin levels upon the completion of successful weight-loss programs, and patients with anorexia nervosa show a decrease of circulating plasma ghrelin following recovery and subsequent weight gain (Druce et al. 2004). Thus, a treatment that acts to artificially lower circulating ghrelin levels has tremendous potential as a tool to halt or even reverse the obesity epidemic as several studies have shown that the suppression of ghrelin or the antagonization of its receptors in the CNS has a powerful ability to promote weight loss (Hu et al. 2005).
Possible pharmaceutical interventions could target ghrelin production or receptor binding. Ghrelin is produced from a precursor called preproghrelin, which can be modified to form several different peptides through gene cleavage or posttranslational processing. Potential pharmaceutical targets for modulating ghrelin activity include both competitive and noncompetitive inhibition of the ghrelin receptor in the hypothalamus.
9.5 Role of Ghrelin in Bariatric Surgery
Bariatric surgery is probably the best therapeutic option for obesity offering long-term benefits in weight control. There are a variety of surgical options available to the bariatric population (Baptista and Wassef 2013). The most commonly employed surgical techniques include the Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, and laparoscopic adjustable gastric banding (AGB). Recent investigations have begun to evaluate the role of ghrelin after bariatric surgery (Pournaras and Le Roux 2009). Despite the exciting results of early studies which demonstrated a significant decrease in plasma ghrelin levels after RYGB surgery, follow-up investigations have had mixed results with studies showing patients having post-procedural ghrelin levels that are decreased, the same, or even increased when compared to preoperative values. The reasons for this heterogeneity in plasma ghrelin levels after RYGB are not entirely clear. It has been suggested that differences in surgical techniques, such as sparing of the vagus nerve, or the creation of vertical (rather than horizontal) gastric pouch, may be at play. Different bariatric surgical techniques have been shown to affect plasma ghrelin levels in different ways, even though they all lead to weight loss in patients (Pournaras and Le Roux 2009). For instance, patients demonstrate a significant decrease in plasma ghrelin levels after sleeve gastrectomy (which removes the gastric fundus), while no such decrease in ghrelin levels is observed in patients after AGB (which preserves the gastric fundus).
Although bariatric surgery can lead to significant weight loss, it is associated with significant morbidity and mortality. The most common complications of bariatric surgery include anastomotic leaks (0.1–5.6 %), intussusception (1 %), gallstones (13–36 %), and operative revisions (39–81 %). Anastomotic leaks can be particularly dangerous resulting in reported mortality rates of 6–50 % (Baptista and Wassef 2013). Given these risks, a less-invasive and less-morbid therapeutic option to treat obese individuals will be more favorable in this population of patients.
9.6 Trans-arterial Embolization of the Left Gastric Artery: Technical Considerations
Percutaneous trans-arterial embolization is a minimally invasive procedure which is routinely performed by interventional radiologists on both an in-patient and out-patient basis, typically to treat a bleeding vessel or to induce ischemia within a tumor. Embolization of the upper GI arteries for bleeding is generally well tolerated given the rich collateral vascular supply to the GI tract. The LGA is a common source of upper GI bleeding and is often prophylactically embolized if no other definitive source for bleeding can be found during angiography. At current, the principles and practices governing the embolization of the LGA for bleeding are similar to those employed in the embolization of the LGA for bariatric purposes. Therefore, the purpose of the following section will be to provide an overview of the vascular supply to the stomach and review the technical aspects involved in LGA embolization.
Vascular Supply to the Stomach
The vascular supply to the stomach is a richly collateralized network of arteries which arises from the three branches of the celiac trunk: the left gastric artery, the common hepatic artery, and the splenic artery (Fig. 9.1). The arteries of the stomach are summarized in Table 9.2.
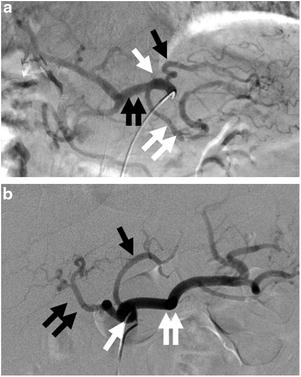

Fig. 9.1
(a) Digital subtraction angiogram (DSA) with a catheter located in the celiac trunk (white arrow) demonstrates the LGA (black arrow), splenic artery (double white arrows), and common hepatic artery (double black arrows). (b) DSA of the celiac trunk (white arrow) in a different patient again shows the LGA (black arrow), splenic artery (double white arrows), and common hepatic artery (double black arrows)
Table 9.2
Summary of the vascular supply to the stomach
Artery | Origina | Vascular territory |
|---|---|---|
Left gastric artery | Celiac trunk | Fundus, proximal lesser curvature |
Right gastric artery | Proper hepatic artery | Pylorus, distal lesser curvature |
Right gastroepiploic artery | Gastroduodenal artery | Distal greater curvature |
Short gastric arteries | Splenic artery
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|