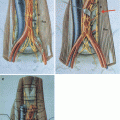
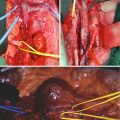
Localization of the tumour
Size
Multiplicity
Extension of the tumour (pT category according to UICC)
Histopathological type (WHO)
In seminoma: presence of syncytiotrophoblasts
In spermatocytic seminoma: any sarcomatous elements
In pluriform tumours: description and percentage of each individual component
Presence of TIN
Immunohistochemistry: AFP and hCG for detection of yolk sac tumour and choriocarcinoma CD31/FVIII for vascular invasion (pluripotency-related markers such as OCT4, NANOG, LIN28, AP-2 gamma for TIN, seminoma, embryonal cell carcinoma)
Table 1.2 shows the different types of germ cell tumours according to the WHO classification.
Histological type | ICD-O-M |
|---|---|
Intratubular germ cell neoplasia, unclassified | 9064/2 |
Others | |
Tumours of one histological type (pure forms) | |
Seminoma | 9061/3 |
(Subtype) Seminoma with syncytiotrophoblastic cells | |
Spermatocytic seminoma | 9063/3 |
(Subtype) Spermatocytic seminoma with sarcoma | |
Embryonal carcinoma | 9070/3 |
Yolk sac tumour | 9071/3 |
Trophoblastic tumours | |
Choriocarcinoma | 9100/3 |
Trophoblastic neoplasms other than choriocarcinoma | |
Monophasic choriocarcinoma | |
Placental site trophoblastic tumour | 9104/1 |
Teratoma | 9080/3 |
Dermoid cyst | 9084/0 |
Monodermal teratoma | |
Teratoma with somatic type malignancies | 9084/3 |
Tumours of more than one histological type (mixed forms) | |
Mixed embryonal carcinoma and teratoma | 9081/3 |
Mixed teratoma and seminoma | 9085/3 |
Choriocarcinoma and teratoma/embryonal carcinoma | 9101/3 |
Others | |
The clinical stage is defined by the UICC TNM classification (Table 1.3). Patients with metastatic disease are classified according to the classification of the International Germ Cell Cancer Collaborative Group (IGCCCG), which also pays regard to the elevation of tumour markers (Table 1.4).
pT | Primary tumour | |||
pTX | Primary tumour cannot be assessed | |||
pT0 | No evidence of primary tumour (e.g. histological scar in testis) | |||
pTis | Intratubular germ cell neoplasia (carcinoma in situ) | |||
pT1 | Tumour limited to testis and epididymis without vascular/lymphatic invasion: tumour may invade tunica albuginea but not tunica vaginalis | |||
pT2 | Tumour limited to testis and epididymis with vascular/lymphatic invasion, or tumour extending through tunica albuginea with involvement of tunica vaginalis | |||
pT3 | Tumour invades spermatic cord with or without vascular/lymphatic invasion | |||
pT4 | Tumour invades scrotum with or without vascular/lymphatic invasion | |||
N | Regional lymph nodes clinical | |||
NX | Regional lymph nodes cannot be assessed | |||
N0 | No regional lymph node metastasis | |||
N1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension or multiple lymph nodes, none more than 2 cm in greatest dimension | |||
N2 | Metastasis with a lymph node mass more than 2 cm but not more than 5 cm in greatest dimension, or multiple lymph nodes, any one mass more than 2 cm but not more than 5 cm in greatest dimension | |||
N3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | |||
pN | Pathological | |||
pNX | Regional lymph nodes cannot be assessed | |||
pN0 | No regional lymph node metastasis | |||
pN1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension and 5 or fewer positive nodes, none more than 2 cm in greatest dimension | |||
pN2 | Metastasis with a lymph node mass more than 2 cm but not more than 5 cm in greatest dimension; or more than 5 nodes positive, none more than 5 cm; or evidence or extranodal extension of tumour | |||
pN3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | |||
M | Distant metastasis | |||
MX | Distant metastasis cannot be assessed | |||
M0 | No distant metastasis | |||
M1 | Distant metastasis | |||
M1a | Non-regional lymph nodes(s) or lung | |||
M1b | Other sites | |||
S | Serum tumour markers | |||
Sx | Serum marker studies not available or not performed | |||
S0 | Serum marker study levels within normal limits | |||
LDH (U/l) hCG (mlU/ml) AFP (ng/ml) | ||||
S1 | <1.5 × N and <5,000 and <1,000 | |||
S2 | 1.5–10 × N or 5,000–50,000 or 1,000–10,000 | |||
S3 | >10 × N or >50,000 or >10,000 | |||
N indicates the upper limit of normal for the LDH assay | ||||
Except for pTis and pT4, where radical orchiectomy is not always necessary for classification purposes, the extent of the primary tumour is classified after radical orchiectomy; see pT in other circumstances. TX is used if no radical orchiectomy has been performed | ||||
According to the 2002 TNM classification, stage I testicular cancer includes the following substages: | ||||
Stage IA | pT1 | NO | MO | S0 |
Stage IB | pT2, pT3, or pT4 | NO | MO | S0 |
Stage IS | Any pT/TX | NO | MO | S1–3 |
Prognosis | 5-year survival | Non-seminoma | Seminoma |
|---|---|---|---|
Good | 90 % | Testis or primary extragonadal retroperitoneal tumour | Any primary localization |
No non-pulmonary visceral metastases | No non-pulmonary visceral metastases | ||
Low markers | Any marker level | ||
AFP <1,000 ng/ml | |||
ß-hCG <1,000 ng/ml (<5,000 IU/l) | |||
LDH <1.5 × normal level | |||
Intermediate | 75 % | Testis or primary extragonadal retroperitoneal tumour | Any primary localization |
No presence of non-pulmonary visceral metastases | Presence of non-pulmonary visceral metastases (liver, CNS, bone, intestinum) | ||
Any marker level | |||
Intermediate markers | |||
AFP 1,000–10,000 ng/ml and/or | |||
ß-hCG 1,000–10,000 ng/ml (5,000–50,000 IU/l) and/or | |||
LDH 1.5–10 × normal level | |||
Poor | 50 % | Primary mediastinal germ cell tumour with or without testis or | – |
Primary retroperitoneal tumour and | |||
Presence of non-pulmonary visceral metastases (liver, CNS, bone, intestinum) and/or | |||
High markers | |||
AFP >10,000 ng/ml and/or | |||
ß-hCG >10,000 ng/ml (50,000 IU/l) and/or | |||
LDH >10 × normal level |
Spiral computerized tomography (CT) scans of the thorax, abdomen and pelvis remain the staging procedures of choice. Magnetic resonance tomography (MRT) can be an alternative, but should be done only at institutions with special expertise in evaluating testis cancer patients [13]. Positron electron tomography (PET) has no role as a staging procedure [14, 15]. Imaging of brain or bones only is mandatory in patients with visceral metastases or in case of symptoms.
AFP, hCG and LDH should be performed before and after orchiectomy. To define a clinical stage I, marker normalization is mandatory. Therefore in case of still elevated markers post-orchiectomy several marker controls might be necessary before finally to define the clinical stage. If markers do not normalize, it is declared as stage IS disease. In patients with metastatic disease, pre-chemotherapy markers instead of pre-orchiectomy markers should be used to allocate the IGCCCG category [12, 16].
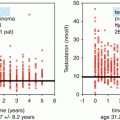
Because further treatment might influence the hormonal status and fertility of the patient a baseline assessment including testosterone, luteinising hormone (LH), follicle-stimulating hormone (FSH) and, if possible, a semen analysis should be performed.
Patients should also be informed about cryoconservation.
1.5 Treatment of Patients with Seminoma CSI
Rete testis infiltration and tumour size >4 cm have been recognized as risk factors for occult retroperitoneal lymph node metastases in a retrospective analysis [17]. A metaanalysis of patients managed by surveillance showed a 5-year relapse rate of 12 % in patients without any risk factor, a relapse rate of 16 % in case of one risk factor and of 32 % in patients with both risk factors. Based on these data, a risk-adapted strategy was recommended with surveillance for patients without any risk factors and adjuvant treatment in those with one or two risk factors. Options for adjuvant therapy are a single course of carboplatin (AUC 7) or paraaortal/paracaval radiation with 20 Gy. Recent data about the induction of secondary malignancies after adjuvant radiotherapy reduced the choice of this strategy [18–20]. Since the latest prospective series from the Canadian Group could not validate the rete testis infiltration and tumour size >4 cm as risk factors, many physicians already favour surveillance independent of any risk factor [21]. On the other hand, a prospective Spanish trial could at least confirm the negative predictive value of these factors [22]. Therefore, no definitive recommendation can be given.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree