The purpose of this article is to provide an update on the current literature evaluating outcomes with laparoscopic prostatectomy. The reported perioperative, oncologic, and functional outcomes with this approach are reviewed and comparisons are made to the open and robotic-assisted approaches.
Key Points
- •
Laparoscopic prostatectomy has perioperative outcomes that are similar to open and robotic-assisted laparoscopic approaches.
- •
Laparoscopic prostatectomy yields short- and intermediate-term oncologic outcomes that are similar to open radical retropubic prostatectomy.
- •
Laparoscopic prostatectomy has functional outcomes (urinary continence and potency) that are similar to open radical retropubic prostatectomy.
- •
Laparoscopic prostatectomy is a more cost-effective minimally invasive approach than a robotic-assisted procedure and can approximate cost equivalence to an open approach.
Prostate cancer is the most common noncutaneous malignancy in men, accounting for 240 890 new cases in 2011. The introduction of serum prostate-specific antigen (PSA) as a screening test in the 1980s aided the detection of prostate cancer and contributed to the increased incidence. Additionally, with the widespread adoption of PSA screening as a public health initiative in the United States, a well-documented stage migration has been noted with a marked increase in the frequency of diagnosis of asymptomatic, clinically localized disease. A population-based analysis has revealed that greater than 90% of newly diagnosed men have localized disease (T1 or T2) that does not extend beyond the capsule of the prostate or involve the seminal vesicles.
There are several treatment options for patients with localized disease, including active surveillance, brachytherapy, radiation therapy, focal therapy (ie, cryosurgery), or surgery. Of these options, level I evidence supporting a survival benefit compared with no treatment is unique to surgery, whereas the other treatments have not been adequately studied in a randomized fashion.
Currently, there are several surgical approaches to radical prostatectomy, including perineal prostatectomy, open radical retropubic prostatectomy, laparoscopic prostatectomy, and robotic-assisted laparoscopic prostatectomy (RALP). In practice, the choice of surgical approach is primarily based on surgeon and patient preference. This review aims to explore the role of laparoscopic prostatectomy in the management of prostate cancer.
History of laparoscopic prostatectomy
Hugh Hampton Young performed the first open prostatectomy for prostate cancer via a perineal incision in 1905. This achievement was followed by the first description of the traditional radical retropubic prostatectomy by Millin in 1947. Several anatomic studies in the 1970s and 1980s allowed for a better appreciation of periprostatic anatomy and consequent reduction in operative morbidity. In an attempt to further decrease operative morbidity and improve postoperative convalescence, minimally invasive techniques were applied to prostatectomy.
Shuessler and colleagues described the initial series of laparoscopic prostatectomy in 1997, reporting on the short-term outcomes of 9 patients. The investigators found that the procedure was feasible, but lengthy operative times (average of 9.3 hours) and postoperative stay (average of 7.3 days) precluded any distinct advantage over open surgery. As a result of these findings and the technical difficulty of a laparoscopic approach, the urologic community did not immediately adopt the procedure.
Interest in laparoscopic prostatectomy was rekindled following the dissemination of the experience from 2 centers in France. Using a reproducible stepwise approach, the operative times in these series were more acceptable (4–5 hours) as were the positive margin rates (15%–28%) and functional outcomes. This technique was subsequently adopted by several centers with surgeons who had the necessary laparoscopic skills.
It is noteworthy that laparoscopic prostatectomy remains a procedure with a challenging learning curve. The number of cases required to become proficient generally ranges from 200 to 700 in the published literature. The learning curve varies for different stages of the procedure, with more challenging steps, such as completion of the vesicourethral anastomosis or optimization of nerve sparing, requiring a higher case volume for proficiency. Attempts to decrease the length of the learning curve have included a rigorous mentored approach as well as completion of a fellowship that encompasses minimally invasive techniques. Others have also found that extensive open prostatectomy experience may not shorten the learning curve for laparoscopic prostatectomy; instead, the curve may be more abbreviated with prior laparoscopic experience.
History of laparoscopic prostatectomy
Hugh Hampton Young performed the first open prostatectomy for prostate cancer via a perineal incision in 1905. This achievement was followed by the first description of the traditional radical retropubic prostatectomy by Millin in 1947. Several anatomic studies in the 1970s and 1980s allowed for a better appreciation of periprostatic anatomy and consequent reduction in operative morbidity. In an attempt to further decrease operative morbidity and improve postoperative convalescence, minimally invasive techniques were applied to prostatectomy.
Shuessler and colleagues described the initial series of laparoscopic prostatectomy in 1997, reporting on the short-term outcomes of 9 patients. The investigators found that the procedure was feasible, but lengthy operative times (average of 9.3 hours) and postoperative stay (average of 7.3 days) precluded any distinct advantage over open surgery. As a result of these findings and the technical difficulty of a laparoscopic approach, the urologic community did not immediately adopt the procedure.
Interest in laparoscopic prostatectomy was rekindled following the dissemination of the experience from 2 centers in France. Using a reproducible stepwise approach, the operative times in these series were more acceptable (4–5 hours) as were the positive margin rates (15%–28%) and functional outcomes. This technique was subsequently adopted by several centers with surgeons who had the necessary laparoscopic skills.
It is noteworthy that laparoscopic prostatectomy remains a procedure with a challenging learning curve. The number of cases required to become proficient generally ranges from 200 to 700 in the published literature. The learning curve varies for different stages of the procedure, with more challenging steps, such as completion of the vesicourethral anastomosis or optimization of nerve sparing, requiring a higher case volume for proficiency. Attempts to decrease the length of the learning curve have included a rigorous mentored approach as well as completion of a fellowship that encompasses minimally invasive techniques. Others have also found that extensive open prostatectomy experience may not shorten the learning curve for laparoscopic prostatectomy; instead, the curve may be more abbreviated with prior laparoscopic experience.
Indications and contraindications
Laparoscopic prostatectomy has identical indications to that of open prostatectomy, primarily being a treatment option offered to patients with organ-confined, nonmetastatic disease. Preliminary results regarding the safety and feasibility of salvage laparoscopic prostatectomy have also been described in several small, single-institution series following radiation therapy or high-intensity focused ultrasound. Because prior treatment can obliterate natural tissue planes and result in periprostatic fibrosis with an attendant increase in potential complications, such as rectal injury, it is advisable that such salvage cases be avoided in a surgeon’s early experience with laparoscopic prostatectomy.
Absolute contraindications to laparoscopic prostatectomy include the conventional contraindications to standard laparoscopy, such as cardiopulmonary insufficiency or coagulopathy. Although additional factors, such as prior abdominal surgery and morbid obesity, are not necessarily contraindications to laparoscopic prostatectomy, they remain important preoperative considerations insofar as they can increase the difficulty of the surgery and increase the risk of open conversion early in a surgeon’s learning curve. Larger prostate glands (>70 g) can also result in increased operative times, blood loss, and postoperative stay.
Instrumentation and approach
The instrumentation ( Table 1 ) required to perform laparoscopic prostatectomy depends to some extent on the surgical approach (transperitoneal vs extraperitoneal) as well as surgeon preference.
| 5-mm trocars (3) 12-mm trocars (3) | Trocar-mounted balloon-dilator device |
| 0° and 30° laparoscope lens, camera holder | Viking 3DHD Vision System (Viking Systems, Westborough, Massachusetts) |
| Suction-irrigation device 10 mm | Monopolar scissors |
| Bipolar forceps: fine and broad | PEER retractor (JARIT Surgical Instruments Inc, Hawthorne, New York) and Endoholder (Codman, Raynham, Massachusetts) |
| Laparoscopic needle drivers (2) | Sutures: 0 polyglactin suture on a CT-1 needle for dorsal venous complex; 2-0 polyglactin suture on an SH needle for bladder neck reconstruction; double-arm 2-0 poliglecaprone on an SH needle for vesicourethral anastomosis |
| Locking, toothed prostate clamp | Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, North Carolina) and Lapra-Ty suture clips (Ethicon Endosurgery, Cincinnati, Ohio) |
| 24F (initial) and 18F (final) urethral catheters |
Extraperitoneal versus Transperitoneal Approach
The extraperitoneal approach offers several potential intraoperative and postoperative advantages and is currently preferred at the authors’ institution. This approach allows patients to remain supine and avoid some complications related to the steep Trendelenburg positioning required with a transperitoneal approach. Intraoperatively, avoiding peritoneotomy allows the peritoneum to contain the bowels and prevents them from obscuring the view of the surgical field. Similarly, this may serve to protect the bowels from inadvertent injury. The extraperitoneal approach also avoids some of the difficulty associated with bowel adhesions from prior surgery, which can complicate a transperitoneal approach. Postoperatively, if a urine leak from the vesicourethral anastomosis should develop, it remains confined to the extraperitoneal space and does not cause peritonitis. Alternatively, if a lymphocele occurs after the extraperitoneal approach, it may be more apt to be symptomatic because the lymphatic fluid is not reabsorbed by contact with the bowel and peritoneal membrane. Although these advantages may come at a potential cost of a decreased operative working space as compared with a transperitoneal approach, a laparoscopic extraperitoneal prostatectomy can still be safely performed in obese patients and/or those with large prostates.
Several retrospective nonrandomized series have compared these approaches and have generally found that operative time and time to return to continence are shorter with the extraperitoneal approach, although these measures are surgeon dependent. Measures of blood loss or transfusion rates, positive surgical margins (PSM), and long-term continence have been similar between the two approaches. In practice, completion of the procedure is feasible and safe with both approaches, and the decision on which approach to use is largely dictated by surgeon preference and prior experience.
Technique
The steps for performing laparoscopic prostatectomy vary slightly between the transperitoneal and extraperitoneal approaches. For the purposes of this review, the operative steps for an extraperitoneal approach are described. Although a formal description of the operative approach is beyond the scope of this review, the procedure can be divided into several reproducible steps.
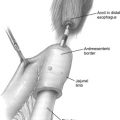
The patient is positioned supine on the operating table. A 1.5-cm midline incision (later used as the extraction incision) is made just caudal to the umbilicus. The incision is deepened through the midline fascia, and the extraperitoneal space is developed with blunt finger dissection, taking care to not enter a plane between the deep inferior epigastric arteries and the undersurface of the rectus muscles. After a sufficient space has been obtained, a 6-trocar technique is used, including two 12-mm trocars and four 5-mm trocars. Under digital guidance, a 5-mm trocar is placed to the left of the incision in the midclavicular line and a 12-mm port is similarly placed to the right. A balloon trocar is placed through the midline incision and insufflation is performed. Two 5-mm trocars are placed near the anterior superior iliac spines bilaterally, and one is placed near the midline between the pubic symphysis and the umbilicus under direct vision ( Fig. 1 ). The surgeon stands on the left side of the patient and the Endoholder (Codman, Raynham, Massachusetts) is anchored to the operating table, allowing for fixed retraction through the left lower quadrant 5-mm trocar and facilitating the remainder of the procedure. Similarly, a camera holder is also fixed to the head end of the operating table to allow for hands-free support of the camera. Both the Endoholder and camera holder free the hands of the surgical assistant and surgeon and expedite the procedure. Additionally, the authors have adopted the use of the Viking 3DHD Vision System (Viking Systems, Westborough, Massachusetts) for this procedure because it facilitates intraoperative vision and can decrease the learning curve of complex laparoscopic procedures.
After establishing insufflation (∼15 mm Hg), bilateral pelvic lymph node dissections are performed. All node-bearing tissue is removed from the obturator fossa and alongside the internal and external iliac arteries and veins bilaterally. Lymphatic attachments are cauterized with bipolar energy and then divided. Care is taken to preserve the obturator nerve and vessels during this dissection. More extensive node dissections are performed for men with intermediate- and high-risk tumors. The endopelvic fascia is then incised sharply and the puboprostatic ligaments are divided, thereby allowing accurate identification of the dorsal venous complex (DVC). The DVC is then suture ligated with a 0-polyglactin suture on a CT-1 needle. The use of a pledget and a Lapra-Ty suture clip (Ethicon Endosurgery, Cincinnati, Ohio) facilitate this because knot tying beneath the pubic symphysis can be time consuming.
The bladder neck is then identified by movement of the Foley catheter and transected widely, distal to the ureteral orifices. Indigo carmine is given earlier in the procedure to help identify the ureteral orifices and verify that iatrogenic injury has not occurred. After division of the posterior bladder neck, the vasa deferentia and seminal vesicles are individually identified, completely mobilized, and divided. Anterior traction on the prostate through the midline 5-mm trocar site and posterior-cranial retraction of the bladder using the left lower quadrant port allows for easier dissection of the seminal vesicles and development of the plane between the prostate and rectum. The prostatic pedicles are controlled with a series of Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, North Carolina) and divided sharply, avoiding cautery given the proximity of the neurovascular bundles. For men with low-risk cancers, the neurovascular bundles are gently mobilized off of the prostate laterally after a high release of levator fascia surrounding the prostate, thereby preserving a veil of tissue (veil of Aphrodite) containing nerves important for erectile function. Classic nerve-sparing or non–nerve-sparing techniques are performed for men with more advanced tumors if the dissection is difficult because of the adherence of the neurovascular bundles to the prostate.
The apical dissection is performed next, which begins with transection of the previously ligated DVC, then the urethra (just distal to the apex of the prostate) and rectourethral muscle. The specimen is then entrapped within a laparoscopic entrapment sac and removed by lengthening the midline fasciotomy. The surgeon can inspect the prostate to determine the adequacy of the dissection while the assistant closes the fasciotomy and reestablishes pelvic insufflation. The wide bladder neck is then reconstructed in a tennis racquet fashion using a 2-0 polyglactin suture on an SH needle, so that the opening in the bladder better approximates the urethral lumen. Finally, the vesicourethral anastomosis is performed using a running double-arm 2-0 polyglyconate suture in the van Velthoven method and secured with Lapra-Ty clips (Ethicon EndoSurgery, Cincinnati, Ohio).
Perioperative outcomes
There are no randomized studies comparing open laparoscopic and robotic-assisted approaches to prostatectomy. Most of the published reports are retrospective single-center case series, which suggest comparable outcomes across these surgical approaches ( Table 2 ). However, comparisons between published series are obscured by differences in patient populations, surgeon experience, and measures of outcome.
| Reference | Approach | No | Mean Operative Time | Mean EBL (mL) | Transfusion (%) | Mean LOS (d) | Complications (%) | PSM |
|---|---|---|---|---|---|---|---|---|
| Lein et al | TLRP | 1000 | 266 | — | 2.2 | — | 11.8 | pT2 14.9 pT3 54.5 pT4 100 |
| Rassweiler et al | TLRP: early group TLRP: late group | 219 219 | 288 218 | 1100 800 | 30.1 9.6 | 12.0 11.0 | 19.6 10.5 | 21.0 23.7 |
| Eden et al | TLRP | 100 | 245 | 313 | 3.0 | 3.8 | 8.0 | 16.0 |
| Guillonneau et al | TLRP | 550 | 200 | 380 | 5.3 | 5.8 | 10.0 | 15.0 |
| Rozet et al | ELRP | 600 | 173 | 380 | 1.2 | 6.3 | 11.5 | 17.7 |
| Ferguson et al | ELRP | 420 | – | 350 | 1.4 | 1.27 | — | pT2 19.1 pT3 44.3 pT4 100 |
| Cohen et al | ELRP | 172 | 170 | 176 | 0 | 1.67 | 9.9 | 14.5 |
| Goeman et al | ELRP | 550 | 188 | 390 | 4.7 | 4.6 | 10.9 | pT2 17.9 pT3 44.8 pT4 71.4 |
| Stolzenburg et al | ELRP | 700 | 151 | 220 | 0.9 | — | 2.4 | pT2 10.8 pT3 31.2 |
| German Group | TLRP (n = 3935) ELRP (n = 1889) | 5824 | 196 | — | 4.1 | — | 8.9 | pT2 10.6 pT3a 32.0 pT3b 56.0 |
| Stolzenburg et al | ELRP | 2000 | 156 | — | 0.6 | 9.0 | Intraoperative: 0.5 Early postoperative: 8.6 Late postoperative: 0.3 | pT2 9.7 pT3 34.4 |
| Rassweiler et al | ORP | 219 | 196 | 1550 | 55.7 | 16.0 | 35.6 | 28.7 |
| Zincke et al | ORP | 3170 | — | 600–1030 | 5.0–31.0 | — | — | 24.0 |
| Lepor et al | ORP | 1000 | — | 819 | 9.7 | 2.3 | 7.0 | 19.9 |
Operative Time
Operative times for laparoscopic prostatectomy by experienced practitioners are generally comparable with open radical retropubic prostatectomy. These times have been shown to consistently decrease with increasing surgical experience across the published literature. At centers of excellence, the operative time has been reported to be in the range of 2.5 to 4.0 hours depending on the experience of the operating surgeon (see Table 2 ).
Intraoperative Blood Loss
A laparoscopic approach offers the benefit of a tamponading effect from the pneumoperitoneum, which is especially relevant when performing a prostatectomy because most of the intraoperative blood loss is related to bleeding from venous sinuses, such as the dorsal venous complex. Additionally, the improved visualization with laparoscopy allows for potentially more meticulous dissection and control of bleeding vessels. In most series, the intraoperative blood loss is 400 mL or less (see Table 2 ) and has been generally found to be less than that seen with open radical retropubic prostatectomy in most comparative studies. A more meaningful parameter may be the transfusion rate, which ranges from 0% to 11% in the published literature (see Table 2 ) and has also been shown to be significantly less than that seen with open radical prostatectomy in many series.
Hospital Stay
A significant difference in hospital stay between a laparoscopic and open prostatectomy has become more difficult to demonstrate because the length of stay has decreased dramatically with open surgery (perhaps in response to shorter initial hospital stays with laparoscopic surgery), with some high-volume centers reporting postoperative stays of 1 to 2 days following open prostatectomy. However, a recent population-based analysis did find that laparoscopic prostatectomy was associated with a statistically significant decrease in length of stay (based on data from 2008). Currently, at the authors’ center (and many others), most patients are routinely discharged on postoperative day 1, with some patients discharged on the same day of surgery.
Oncologic outcomes
Postoperative oncologic control can be evaluated using surrogate measures, such as the absence of a PSM or freedom from biochemical recurrence (BCR) (PSA recurrence) in series in which the follow-up is not mature enough to yield more conventional outcomes, such as cancer-specific and overall survival. Many laparoscopic prostatectomy series have limited follow-up because the procedure began to gain wider use only after the year 2000.
PSM Rate
The overall PSM rate for laparoscopic prostatectomy ranges from 11% to 31% (see Table 2 ; Table 3 ). The rate of PSM is dependent on surgeon experience, disease characteristics of the patient cohort, and pathologic processing of the specimen. In comparative studies, there is only one randomized trial and one prospective study. In the randomized trial, which included a single surgeon and 120 patients, no difference was found in PSM rates between the open and laparoscopic approaches for the overall cohort (26% vs 22%, P = .28), those with organ-confined disease, and those with extraprostatic extension of disease. Similarly, no differences were found in the prospective trial when the PSM rates were stratified by pathologic stage. A systematic review of the literature also found no difference in PSM rates between open and laparoscopic approaches. Similarly, no difference in PSM rates has been observed in comparative studies between laparoscopic and robotic-assisted approaches.