Gastric cancer is common worldwide. Tumor location and disease stage differ between Asian and Western countries. Western patients often have higher BMIs and comorbidities that may make laparoscopic resections challenging. Multiple trials from Asian countries demonstrate the benefits of laparoscopic gastrectomy for early gastric cancer while maintaining equivalent short-term and long-term oncologic outcomes compared with open surgery. The outcomes of laparoscopy seem to offer equivalent results to open surgery. In the United States, laparoscopic gastrectomy remains in its infancy and is somewhat controversial. This article summarizes the literature on the epidemiology, operative considerations and approaches, and outcomes for laparoscopic gastrectomy.
Key Points
- •
Differences between Eastern and Western countries exist in both location and stage of gastric cancer at presentation. In addition, patients in the West tend to be, on average, older, have a higher BMI, and have more extensive comorbidities.
- •
Staging laparoscopy is done with adjunct studies such as peritoneal cytology and intraoperative ultrasound, which are valuable tools in the diagnostic evaluation of patients with potentially resectable disease.
- •
Laparoscopic gastrectomy is a challenging operation; advanced laparoscopic skills are required to perform an adequate lymphadenectomy and an intestinal anastomosis in an oncologically appropriate manner.
- •
Numerous studies from Asia have demonstrated the benefits of laparoscopic gastrectomy for malignancy while maintaining equivalent short-term and long-term oncologic outcomes compared with open surgery. In the United States, further experience and standardization are needed before these techniques will become widely accepted.
- •
As surgeons continue to investigate minimally invasive techniques for gastric cancer, new technologies may be developed that shorten the extensive learning curve required to master these complex procedures.
Introduction
Laparoscopic gastrectomy for gastric cancer was pioneered in Eastern Asia, where the incidence of gastric cancer is high and population-based screening programs exist. In 1994, Kitano and colleagues performed the first laparoscopic-assisted distal gastrectomy with lymph node dissection for gastric cancer. Since that time there has been a plethora of data from eastern Asia demonstrating the safety, feasibly, and oncologic outcomes of laparoscopic gastrectomy. In Western countries, Belgium and Italy have been on the forefront of advancing laparoscopic gastrectomy for malignancy. In 1993, Azagra and colleagues performed the first laparoscopic distal gastrectomy with Billroth II anastomosis for gastric cancer and, in 1995, this group reported the first laparoscopic total gastrectomy for cancer. Huscher and colleagues, from Italy, reported the only prospective, randomized trial to date in the West regarding 5-year clinical outcomes of laparoscopic-assisted subtotal gastrectomy compared with open surgery for stage-matched adenocarcinomas, demonstrating both safety and feasibility of the laparoscopic approach. Five-year survival numbers showed no significant difference between the two groups, but patients in the laparoscopic group had less blood loss, earlier oral intake and shorter hospital stay.
In the United States, the role of laparoscopy for the treatment of cancer has evolved considerably, but at a much slower pace. The short-term benefits of laparoscopy compared with laparotomy are apparent, but the main concerns have centered on oncologic equivalency. Specifically, these issues include recurrence and survival, the ability to laparoscopically obtain negative margins and perform an adequate lymph node dissection, the potential for local tumor dissemination and port-site metastasis, and the effects of CO 2 pneumoperitoneum. As these issues have been addressed by studies in support of laparoscopic surgery for colorectal cancer, the use of laparoscopy for malignancy has increased. However, in the United States, there are few data regarding the role of laparoscopic gastrectomy in the treatment of gastric carcinoma. Literature from the United States contains only five retrospective comparative studies, with the largest report including only 30 patients in the laparoscopic group. This may be related to the lower incidence of gastric cancer in the United States and the higher proportion of locally advanced disease at diagnosis. In addition, there is a significant learning curve for these advanced laparoscopic procedures. As such, in the United States, the use of laparoscopic gastrectomy for the treatment of gastric cancer is low and remains somewhat controversial. However, as investigation of advanced laparoscopy for gastric cancer continues and results of randomized trials become available, surgeons will gain more experience and techniques will become more standardized and broadly accepted. Currently laparoscopic gastrectomy for gastric cancer should be considered only in select cases. Patients with early mucosal disease seem to be good candidates for a laparoscopic approach given the low (<3%) risk of nodal disease. In addition, patients with advanced disease may be suitable candidates for a laparoscopic palliative resection. However, for most patients in the United States who present with locally advanced disease, laparoscopic gastrectomy should be recommended only within the confines of investigational trials at centers experienced in treating patients with gastric cancer.
Introduction
Laparoscopic gastrectomy for gastric cancer was pioneered in Eastern Asia, where the incidence of gastric cancer is high and population-based screening programs exist. In 1994, Kitano and colleagues performed the first laparoscopic-assisted distal gastrectomy with lymph node dissection for gastric cancer. Since that time there has been a plethora of data from eastern Asia demonstrating the safety, feasibly, and oncologic outcomes of laparoscopic gastrectomy. In Western countries, Belgium and Italy have been on the forefront of advancing laparoscopic gastrectomy for malignancy. In 1993, Azagra and colleagues performed the first laparoscopic distal gastrectomy with Billroth II anastomosis for gastric cancer and, in 1995, this group reported the first laparoscopic total gastrectomy for cancer. Huscher and colleagues, from Italy, reported the only prospective, randomized trial to date in the West regarding 5-year clinical outcomes of laparoscopic-assisted subtotal gastrectomy compared with open surgery for stage-matched adenocarcinomas, demonstrating both safety and feasibility of the laparoscopic approach. Five-year survival numbers showed no significant difference between the two groups, but patients in the laparoscopic group had less blood loss, earlier oral intake and shorter hospital stay.
In the United States, the role of laparoscopy for the treatment of cancer has evolved considerably, but at a much slower pace. The short-term benefits of laparoscopy compared with laparotomy are apparent, but the main concerns have centered on oncologic equivalency. Specifically, these issues include recurrence and survival, the ability to laparoscopically obtain negative margins and perform an adequate lymph node dissection, the potential for local tumor dissemination and port-site metastasis, and the effects of CO 2 pneumoperitoneum. As these issues have been addressed by studies in support of laparoscopic surgery for colorectal cancer, the use of laparoscopy for malignancy has increased. However, in the United States, there are few data regarding the role of laparoscopic gastrectomy in the treatment of gastric carcinoma. Literature from the United States contains only five retrospective comparative studies, with the largest report including only 30 patients in the laparoscopic group. This may be related to the lower incidence of gastric cancer in the United States and the higher proportion of locally advanced disease at diagnosis. In addition, there is a significant learning curve for these advanced laparoscopic procedures. As such, in the United States, the use of laparoscopic gastrectomy for the treatment of gastric cancer is low and remains somewhat controversial. However, as investigation of advanced laparoscopy for gastric cancer continues and results of randomized trials become available, surgeons will gain more experience and techniques will become more standardized and broadly accepted. Currently laparoscopic gastrectomy for gastric cancer should be considered only in select cases. Patients with early mucosal disease seem to be good candidates for a laparoscopic approach given the low (<3%) risk of nodal disease. In addition, patients with advanced disease may be suitable candidates for a laparoscopic palliative resection. However, for most patients in the United States who present with locally advanced disease, laparoscopic gastrectomy should be recommended only within the confines of investigational trials at centers experienced in treating patients with gastric cancer.
Epidemiology
Gastric cancer is the fourth most commonly diagnosed malignancy worldwide, with nearly 990,000 cases per year. Approximately 70% of these cases occur in developing countries. It is the second leading cause of cancer mortality with an estimated 738,000 deaths per year. The highest incidence rates are in Eastern Asian countries, with a male predominance of 2 to 1. In the United States, there are approximately 21,300 cases diagnosed and more than 10,300 deaths yearly. Despite advances in multimodality therapy for gastric cancer, recurrence and mortality remain high. In the United States, the overall 5-year survival for all stages combined is 28%. Approximately 95% of cases are adenocarcinomas, with the remaining 5% being a mix of lymphoma, carcinoid, leiomyosarcoma, gastrointestinal stromal tumors, adenosquamous, and squamous cell carcinomas.
Differences between Eastern and Western hemisphere countries exist in both location and stage of disease at presentation. In Eastern countries, like Japan and South Korea where much of the data related to laparoscopic gastric surgery originate, cancers tend to be in the middle and distal portions of the stomach. In the West, however, carcinomas of the proximal stomach are increasingly more common. Histologically, intestinal type tumors predominate in the East, while in the West the predominant type is diffuse. In addition, Eastern countries have intense screening programs that detect up to 70% of cancers at an early stage. In contrast, in Western nations most lesions are detected only after causing symptoms such as pain, dysphagia, and anemia, and, as a result, up to 80% of patients will already have advanced disease at diagnosis. Furthermore, patients in the West tend to be an average of 10 years older (average age 70 in the United States), significantly more overweight, have a higher incidence of cardiovascular disease, and a higher risk of thromboembolic complications.
Diagnostic evaluation
Proper staging of patients with gastric cancer is essential for providing the appropriate treatment course and is based on the American Joint Committee on Cancer system, which includes characteristics of the tumor (T), nodal status (N), and metastatic disease (M). A separate Japanese classification system exists but is not as broadly used, especially in the United States. Esophagogastroduodenoscopy and tissue biopsy are essential for pathologic diagnosis. Evaluation should include CT scans of the abdomen and pelvis and, for proximal lesions, CT scan of the chest. Although CT has limited accuracy for assessing the depth of tumor invasion or regional nodal involvement, it may detect distant nodal or visceral metastases, ascites, or carcinomatosis. However, preoperative CT scans often underestimate the extent of disease, principally because of radiographically undetectable metastases involving the liver and peritoneum. Endoscopic ultrasonography (EUS) should also be used to provide a more accurate staging evaluation of the T and N status. EUS has a T-stage accuracy of 60% to 80% and a lymph node accuracy of 50% to 65%. Additionally, EUS allows for fine-needle aspiration of suspicious lymph nodes and can detect liver metastases missed by conventional imaging studies. As such, EUS is important in identifying lesions that may be amenable to endoscopic mucosal resection and selecting patients for neoadjuvant therapy. PET scanning may be used as adjunct imaging to detect distant metastases, but approximately 50% of diffuse type tumors are not avid for the principal tracer, fluorodeoxyglucose F 18.
Staging laparoscopy, though more invasive than CT or EUS, has the advantage of directly visualizing the liver surface, peritoneum, and local lymph nodes, and permits biopsy of any suspicious lesions. Furthermore, the stomach can be closely inspected for direct tumor extension into surrounding organs such as the liver, pancreas, colon, and spleen. Bulky nodal disease does not necessarily preclude resection, but lymph nodes outside the planned resection bed or suspicious nodules should be sent for intraoperative frozen section analysis. At laparoscopy, radiologically occult metastases are documented in up to 40% of patients with gastric cancer and a negative CT, who would have been considered potentially resectable. Power and colleagues found that those with T1–2, N0 tumors on EUS were at much lower risk for metastasis at laparoscopy than those with T3–4 or N+ disease (4% vs 25%). In addition, laparoscopic ultrasound is a valuable adjunct to detect and evaluate liver metastases that are not visualized from the surface.
Peritoneal cytology can also be obtained during staging laparoscopy. This is performed by instilling 100 to 200 cc of normal saline into the pouch of Douglas and the paracolic gutters. At least 50 cc should be recovered for cytologic analysis. Patients with peritoneal cytology that is positive for malignant cells (up to 60% of patients with locally advanced disease on imaging) should be treated as having metastatic disease because their survival following resection is similar to those with overt metastases. In select cases, patients with positive peritoneal cytology may be reevaluated for resection after chemotherapy, if they demonstrate no evidence of disease progression.
For surgeons who routinely perform open gastrectomy for gastric cancer, the selection of patients who need staging laparoscopy is controversial. Guidelines from the National Comprehensive Cancer Network suggest only that laparoscopy be considered for patients who seem have advanced locoregional disease (T3–4 or N+) after conventional radiographic and EUS staging. The authors’ practice is to perform staging laparoscopy with peritoneal cytology for any medically fit patient with locally advanced gastric cancer who does not have histologic confirmation of stage IV disease. Following completion of staging laparoscopy, if the cancer remains resectable with a curative intent, the decision is then made whether to proceed with a laparoscopic gastrectomy or convert to an open procedure.
Early gastric cancer
A thorough understanding of the presentation, natural history, and biologic behavior of gastric cancer, as well as the accurate location and staging of the disease, is critical for providing an oncologically appropriate and safe operation. The extent of surgical resection is determined by the tumor size, depth of invasion, and extent of lymph node involvement. Early gastric cancer (EGC) is defined as a tumor that invades no more than the submucosa (T1). Lesions limited to the mucosa (carcinoma in situ [Tis]–T1a) have a reported rate of regional lymph node metastasis of 1% to 3%, in contrast to lesions that penetrate the submucosa (T1b), which have a rate of 8%–18%, depending on tumor size. Endoscopic mucosal resection and endoscopic submucosal dissection have gained notoriety in Asian countries for patients with EGC and a low risk of lymph node involvement. In Western countries there has been less experience with these techniques, likely owing to the differences in stage at presentation. Current guidelines in the United States limit endoscopic resection to Tis or well to moderately differentiated mucosal lesions (T1a) with no evidence of ulceration, lymphovascular invasion, or lymph node metastases. Alternatively, lesions limited to the mucosa (Tis or T1a) can be resected by laparoscopic wedge resection. Preoperative tattooing of the lesion and intraoperative endoscopy are helpful for intraoperative localization. Laparoscopic wedge resection can then be completed with laparoscopic reticulating stapling devices. Careful inspection of the specimen should be made to exclude positive margins. One study reported an 88% 5-year survival, but 26% of patients were found to have submucosal invasion (T1b) on pathologic examination, stressing the importance of accurate preoperative T-staging. Thus, these patients must be followed closely with diligent surveillance for recurrence of disease. T1b lesions are best treated with formal gastric resection. These lesions are well suited for a laparoscopic approach and, in fact, most of the literature regarding laparoscopic gastrectomy for cancer concerns early stage disease.
Locally advanced gastric cancer
Owing to the increased risk of lymph node involvement and metastatic disease for advanced tumors (≥T3), surgeons were initially cautious with using laparoscopic techniques for advanced gastric cancer. Until recently, investigators recommended against a laparoscopic approach for cancer that was preoperatively staged as more advanced than T2N1. However, because so few patients present with EGC in the West, several investigators have recently studied laparoscopic resection for advanced gastric cancer with the hopes of extending the potential benefits of laparoscopy to a larger patient population. The increased complexity and technical difficulty of these operations is related to the greater nodal burden of disease as well as the potential for adherence to surrounding organs, namely the pancreas, spleen, and liver. Initially, there was hesitancy on the part of surgeons to perform these operations laparoscopically without evidence for oncologic equivalency to open procedures. However, as experience with laparoscopic gastric resection has grown, some investigators have begun to expand these techniques to more advanced disease and have demonstrated comparable short-term and long-term outcomes to open resections.
Extent of lymph node dissection
One of the most controversial areas in the surgical management of gastric cancer is the optimal extent of lymph node dissection. Three types of lymph node dissection are performed: perigastric (D1 + α), additional dissection along the common hepatic artery (D1 + β), and extended dissection of nonregional lymph nodes (D2). Eastern Asian surgeons routinely perform extended lymphadenectomy (D2), a practice that some suggest at least partially accounts for the better survival rates in Asian compared with Western series. However, Western studies, including two large, prospective, randomized trials have failed to show a survival benefit with extended lymph node dissection. In addition, these trials demonstrated increased postoperative morbidity and mortality associated with more extensive dissections. As a result, an extended lymphadenectomy is typically not performed in most Western countries. In the United States, it is generally accepted to perform an en bloc resection of the regional lymph nodes with the goal of obtaining a minimum of 15 lymph nodes.
Operative considerations for formal gastric resection
Tumor Location
In general, tumors located in the distal two-thirds of the stomach are best treated with subtotal gastrectomy; whereas, tumors of the proximal one-third of the stomach are treated with total gastrectomy. In Eastern Asia, in an effort to improve the quality of life after gastrectomy, various types of function-preserving laparoscopic gastrectomies have been described for the treatment of EGC. These include proximal gastrectomy, pylorus preserving gastrectomy, and vagus nerve preserved gastrectomy. Laparoscopic proximal gastrectomy has been used to treat ECG tumors located in the proximal third of the stomach. However, this procedure requires an esophagogastrostomy, which is associated with a high rate of gastroesophageal reflux. Laparoscopic pylorus preserving gastrectomy with gastrogastrostomy has been used to treat ECG tumors located in the body of the stomach. This technique has been demonstrated to have satisfactory long-term surgical and oncologic outcomes compared with an open procedure, with potentially lower rates of postgastrectomy dumping syndrome and biliary reflux compared with distal gastrectomy. Technical modifications for this procedure include excluding the removal of the prepyloric lymph nodes, assurance of a 4-cm distal margin from the tumor, and a 3- to 4-cm antral cuff for an adequate gastrogastric anastomosis. Given the rarity of ECG in the United States, as well as the limited postoperative data, laparoscopic function-preserving gastrectomies currently have very minimal application in the United States.
Approach
The decision to use a laparoscopic versus an open approach depends on several factors. Principal considerations relate to the technical expertise and experience of the surgeon with advanced minimally invasive techniques. Laparoscopic gastrectomy is a technically challenging operation that requires the skills to perform an adequate lymphadenectomy and an intestinal anastomosis. Relative contraindications for a surgeon who is early in their learning curve include patients with high body mass index (BMI) and advanced tumors, especially those invading surrounding organs requiring en bloc resection. Various technical disadvantages for laparoscopic resection in obese patients have been reported and include decreased surgical visibility, a dissection plane hindered by adipose tissue, and difficulty with anastomoses. Higher BMI has also been reported as an independent risk factor for increased risk of pancreatic fistula following laparoscopic distal gastrectomy. Furthermore, patients with multiple severe comorbidities, those who have received neoadjuvant therapy, or those who have undergone previous laparotomy may not be good candidates for laparoscopic resection early in a surgeon’s learning curve. An absolute contraindication is poor cardiopulmonary reserve, due to the decrease in venous return and increase in pulmonary resistance associated with pneumoperitoneum.
Hand-assisted laparoscopic gastrectomy has been advocated by some groups of investigators to overcome the technical challenges associated with a totally laparoscopic approach. Laparoscopic gastrectomy is well suited for a hand-assisted approach because an extraction incision is already needed to remove the specimen. Hand-assisted laparoscopic gastrectomy has been shown to maintain the advantages of laparoscopy (less pain, quicker recovery, and shorter hospital stay) while decreasing operative time and minimizing conversions to laparotomy. As with any cancer operation, the safety of the patient and the ability to complete an oncologically sound resection are of paramount concern. Surgeons attempting laparoscopic gastrectomy should have a low threshold for conversion to an open procedure.
Oncologic Concerns
Given the paucity of oncologic outcome data in the United States regarding laparoscopic gastrectomy for gastric cancer, there continues to be significant controversy. As such, caution and meticulous laparoscopic technique must be exercised to adhere to the strict principles of oncologic surgery in patients with potentially curative resections. These principles include minimal tumor manipulation, obtaining negative margins, and performing an adequate lymph node dissection. There also remain questions regarding trocar-site metastasis and the effects of CO 2 pneumoperitoneum. However, there is no good experimental evidence suggesting that CO 2 pneumoperitoneum increases the malignant potential of cancer cells. In addition, it has been reported that incisional tumor metastases can occur in 1% of patients with colon cancer following traditional laparotomy, but data exist regarding this risk in gastric cancer. Tumor dissemination and trocar-site metastasis are most likely related to poor technique or advanced disease. Specific technical steps that can be used to prevent trocar site metastasis include (1) minimize handling of the tumor, (2) secure trocars to skin with suture to prevent dislodgement, (3) irrigate the trocar sites with a tumoricidal agent, and (4) use a wound protector at the extraction site. Importantly, the laparoscopic approach must provide oncologic equivalency and must not be a compromise or short-cut procedure.
Patient position and room setup
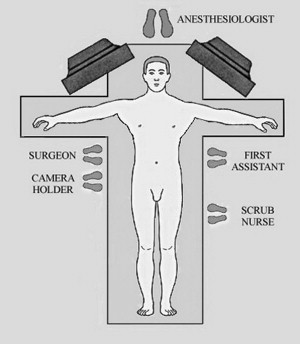
The patient is placed in the supine position with both arms extended ( Fig. 1 ). General endotracheal anesthesia is administered. An orogastric tube is placed to decompress the stomach and a Foley catheter is inserted into the bladder. Intravenous antibiotics are administered within 1 hour of the incision. Sequential stockings are applied to the lower extremities for deep venous thrombosis prophylaxis. Additionally, the authors give all patients 5000 units of subcutaneous unfractionated heparin in the preoperative holding area. The patient is secured to the surgical bed to facilitate maximum reverse Trendelenburg position. Additionally, either a bean bag and/or foot board should be used. The video monitors are positioned near the shoulders on each side of the operating table. The surgeon and camera holder stand on the patient’s right side. The first-assistant and scrub nurse are positioned on the left side.
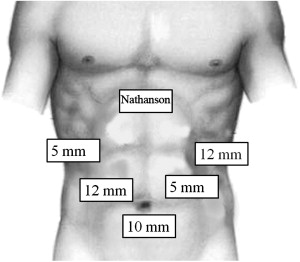
The initial trocar is placed approximately 15 cm below the xiphoid in the midline ( Fig. 2 ). This can be performed using either a Veress needle or an open Hasson technique, depending on the surgeon’s preference and experience. An angled telescope (30° or 45°) should be used to facilitate looking around the corners and to maximize the field of view. Under direct laparoscopic visualization, two additional trocars (5-mm and 12-mm) are placed on each side of the abdomen. A Nathanson liver retractor (Cook Medical, Bloomington, IN, USA) is placed directly through the abdominal wall in the subxiphoid location. The liver retractor is positioned to retract the left lateral segments of the liver to expose the diaphragmatic hiatus. The 12-mm right-sided trocar is the primary operative port and is used to introduce the laparoscopic linear stapler, as well as the EndoStitch (Covidien, Norwalk, CT, USA) or needle holder. The patient should be positioned in steep reverse Trendelenburg to facilitate exposure of the proximal stomach and the diaphragmatic hiatus.
Operative technique
Laparoscopic Subtotal Gastrectomy
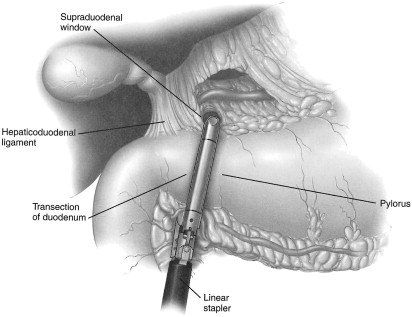
The first step is to perform a staging laparoscopy (see above) to asses for the potential of curative resection. If the cancer is resectable, the decision is then made as to the feasibility of performing the operation laparoscopically. The laparoscopic procedure begins by retracting the greater omentum cephalad and releasing the omentum from the transverse colon. This is performed with ultrasonic coagulating shears and allows entry into and visualization of the lesser sac. The right gastroepiploic pedicle is followed toward the patient’s right side. The pedicle is clipped and ligated close to its origin from the gastroduodenal artery. Attention is then turned to the prepyloric lymph nodes, which are carefully dissected. The proximal duodenum is dissected circumferentially. This is facilitated by the assistant retracting the antrum laterally to linearize the first portion of the duodenum. Careful circumferential retroduodenal dissection just proximal to the duodenal fusion with the head of the pancreas is performed. The duodenum is transected with a reticulating linear 60-mm laparoscopic stapler with a 3.5-mm staple load ( Fig. 3 ). This allows for greater mobility and visualization of the right gastric artery and the lymph nodes of the perigastric region and lesser curve. The right gastric artery and associated lymph nodes are divided close to its origin from the hepatic artery proper. If indicated, depending on tumor location, the left gastric vessels are divided near their respective roots with a linear laparoscopic stapler with a vascular cartridge. The proximal site of gastric transection is determined by the location of the tumor. A minimum of 4-cm is needed to assure an adequate proximal margin. As such, for more proximal lesions, it may be necessary to divide the left gastroepiploic artery near its origin, as well as some of the short gastric vessels. The stomach is then transected with multiple (typically 3 or 4) applications of the laparoscopic linear stapler. A 3.5-mm cartridge can often be used in the proximal stomach, whereas a 4.8-mm load may be required for the thicker distal portions. It is important to remove the orogastric tube from the stomach before transection. The specimen is placed in a retrieval bag for later removal.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



