The laparoscopic approach for benign and malignant lesions in the tail of the pancreas is becoming a more widely used approach. Multiple prospective studies have shown the feasibility and safety of laparoscopic distal pancreatectomy in single-center and multi-center settings. Laparoscopic distal pancreatectomy is a challenging procedure, because the pancreas is surrounded by critical structures and located in the retroperitoneum. Pancreatic fistula remains a common complication in the laparoscopic approach. Distal pancreatic aggressive tumors may not be appropriate for the laparoscopic approach due to the lack of oncologic safety studies.
Key Points
- •
Laparoscopic distal pancreatectomy (LDP) is a safe procedure with a morbidity rate comparable to that of open distal pancreatectomy (ODP).
- •
Pancreatic surgeons should report their outcome diligently (resection margin, lymph node count, and morbidity rate, including pancreatic fistula), and survival with the importance of following International Study Group on Pancreatic Fistula (ISGPF) guidelines.
- •
Selective ligation of the main pancreatic duct remains a challenging step in the laparoscopic approach and it may decrease fistula rate.
- •
The role and oncologic safety of LDP for pancreatic adenocarcinoma or mucinous cystadenocarcinoma remain unknown.
- •
LDP with spleen preservation can be considered a matter of surgical preference and is not recommended in cases of suspected or confirmed adenocarcinoma of the body/tail of the pancreas or in cases of splenic vessels encasement.
Introduction
Laparoscopic approach for benign and malignant lesions in the tail of the pancreas is becoming a more widely used approach. Multiple prospective studies have shown the feasibility and safety of LDP in single-center and multicenter settings. Distal pancreatectomy (DP) is defined as the resection of the body/tail of the pancreas to the left of the superior mesenteric vein. LDP is a challenging procedure, because the pancreas is surrounded by critical structures and located in the retroperitoneum. Mishandling the pancreas itself can be unforgiving and may result in iatrogenic injury that may increase the risk of complications, such as pancreatic fistula. Distal pancreatic adenocarcinoma is an aggressive tumor and may not be appropriate for the laparoscopic approach, especially because many pancreatic surgeons are not taught to perform advanced laparoscopy during their residency training.
Introduction
Laparoscopic approach for benign and malignant lesions in the tail of the pancreas is becoming a more widely used approach. Multiple prospective studies have shown the feasibility and safety of LDP in single-center and multicenter settings. Distal pancreatectomy (DP) is defined as the resection of the body/tail of the pancreas to the left of the superior mesenteric vein. LDP is a challenging procedure, because the pancreas is surrounded by critical structures and located in the retroperitoneum. Mishandling the pancreas itself can be unforgiving and may result in iatrogenic injury that may increase the risk of complications, such as pancreatic fistula. Distal pancreatic adenocarcinoma is an aggressive tumor and may not be appropriate for the laparoscopic approach, especially because many pancreatic surgeons are not taught to perform advanced laparoscopy during their residency training.
Diagnosis and staging
Early reports of laparoscopic pancreas resections focused on staging. The use of pancreatic protocol CT scan, has limited the need for staging laparoscopy. Staging laparoscopy using intraoperative ultrasonography may assess vascular tumor involvement and local respectability but no comparative data exist to describe the advantages of this approach over staging CT scan.
A tissue diagnosis is required in specific cases where preoperative or palliative chemotherapy is considered part of the treatment of pancreatic cancer. Endoscopic ultrasonography fine-needle aspiration (EUS-FNA), cystic fluid sampling, and endoscopic retrograde cholangiopancreatography (ERCP) are the most common options for pancreatic tissue biopsy. ERCP may be useful for evaluating the ductal anatomy and its relation to the lesion. Alternatively, magnetic resonance cholangiopancreatography is a noninvasive study to evaluate the pancreatic anatomy. EUS-FNA is the most reliable and accurate method of obtaining tissue. Lennon and colleagues described their EUS-guided tattooing technique before LDP. EUS-guided tattooing was feasible in all 13 cases (EUS-guided tattooing to surgery, mean 20.3 days). The tattoo was visible in all cases with no significant complications associated with this technique. Newman and colleagues reviewed the safety and efficacy of preoperative EUS-guided tattooing. Ten patients underwent preoperative EUS-guided tattoo and the lesions were identified intraoperatively. The nontattoo group (26 patients) had variable success and 1 patient required a repeat operative procedure because of inability to identify 8-mm insulinoma. More studies are needed to identify the role of EUS-guided tattooing. The potential of decreasing operative time and demarcating a precise line of resection for laparoscopic surgeons makes this procedure attractive for small pancreatic lesions.
Selection criteria
LDP for appropriate distal pancreatic neoplasms includes all benign lesions and neoplasms without metastasis or local invasion (ie, colon or stomach invasion or encasement of adjacent major vessels, such as common hepatic artery, superior mesenteric artery, celiac axis, and portal vein).
In general, patients should be less than 80 years of age and have been diagnosed with lesions in the pancreatic body/tail localized pancreatic cancer (≤T3) without evidence of distant metastasis, peritoneal seeding, or para-aortic lymph node metastasis. Cases with high malignant potential, such as invasive ductal cancer and mucinous cystic neoplasm, should be treated with en bloc resection of the spleen. Islet cell tumor and cystic diseases other than mucinous cystic neoplasm can be treated with spleen-sparing LDP. Patients needing venous resection were considered candidates for resection. In addition, surgery may be appropriate when R0 resection is expected from combined resection of adjacent organs, such as stomach, transverse colon, left adrenal gland, and left kidney.
Tumor biology
Ultimate success of any oncologic surgery depends on cancer-related survival. This specific oncological outcome is mainly driven by tumor biology, tumor margins, and adequacy of lymph node dissection.
Cystic Pancreatic Neoplasms
Most cystic neoplasms of the pancreas are benign but range from completely benign simple cysts, serous cystadenomas, and pseudocysts to potentially premalignant mucinous cystadenomas and intraducal pappilary mucinous neoplasms (IPMNs) to malignant IPMNs and malignant mucinous cystadenocarcinomas. Large neoplasm size, presence of symptoms, rapid tumor growth, and cyst fluid carcinoembryonic antigen level greater than 200 ng/mL should prompt surgery. A crucial point in LDPs for cystic neoplasm is tumor manipulation and potential rupture, which may be under-reported.
Neuroendocrine Tumors
Pancreatic neuroendocrine tumors are frequently identified incidentally. They are rare neoplasms with an incidence of 1 per 100,000. Pancreatic neuroendocrine tumors can be functional or nonfunctional. They can manifest in association with an inherited syndrome, such as multiple endocrine neoplasia, or sporadically. Song and colleagues evaluated 359 patients who underwent LDP; 10% had neuroendocrine tumors. Fernandez-Cruz and colleagues reported 49 patients with neuroendocrine tumors; 51% had LDP and 48% had laparoscopic enucleations with negative resection margins in all malignant lesions.
Pancreatic Ductal Adenocarcinoma
Adequate lymphadenectomy provides useful staging to influence decisions regarding adjuvant chemotherapy or chemoradiation therapy. The ratio of positive to procured lymph nodes has been shown of prognostic significance. The adequacy of lymph node dissection during LDP has been under investigation. The mean number of lymph nodes harvested has been reported between 10.3 ± 8.6 and 18 ± 4.
Resections
The 3 most common types of distal pancreatic resections performed for neoplastic disease are LDP, laparoscopic spleen-preserving DP, and laparoscopic tumor enucleation of the distal pancreas.
LDP is the most common laparoscopic pancreatic resection. Gagner and colleagues published the first 8 LDPs for islet cell tumors. Recently, large series were published showing comparative perioperative results with ODP. Common variations include spleen-preserving LDP with or without splenic vessels preservation, LDP with splenectomy, and the radical antegrade modular pancreatectomy.
Preoperative Considerations
If splenectomy is anticipitated, then vaccination against encapsulated bacteria ( Haemophilus influenza, Streptococcus, and Meningococcus) should be given 7 to 10 days before LDP. Patients with functional pancreatic neuroendocrine tumors require physiologic status optimization before surgery. Preoperative antibiotics and deep venous thrombosis prophylaxis are provided according to Surgical Care Improvement Project guidelines.
Positioning of the Patient, Operator, and Trocars
Patients are placed in the right lateral decubitus position using beanbags or large gel rolls. This position allows gravity to play a retractor role during the mobilization. A nasogastric tube and a Foley catheter are usually placed after anesthesia induction.
The operating surgeon and the second assistant stand to the right of the patient, and the first assistant with the scrub nurse stand on the left side of the patient.
Pneumoperitoneum is established via open technique (through perimumbilical incision with 12-mm trocar under direct vision) or closed technique (Veress needle in the left upper quadrant). Abdominal pressure is maintained at 15 mm Hg by insufflation of carbon dioxide. Four trocars, 2 5-mm trocars (1 in the left flank and 2 in the epigastrium) and 2 12-mm trocars (1 in the perimumbilical region for the scope and 1 in the midclavicular line at the same level of the umbilicus), allow the procedure to progress. An additional 5-mm subxiphoid trocar is occasionally added to help in retracting the left lobe of the liver (Nathanson retractor). Surgeons should triangulate their trocars around the body/tail of the pancreas with at least 5 cm distance that allows sufficient range of motion.
Laparoscopic distal pancreatectomy with en bloc splenectomy
Several technical variations have been described in the literature based on surgeon preference. The authors prefer the following technique.
Lesser Sac Exposure and Splenic Flexure Mobilization
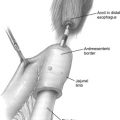
After exploring the abdominal cavity for any metastases, the gastrocolic ligament is divided using LigaSure (Valleylab, Boulder, CO, USA) to open the lesser sac and to expose the plane between the posterior wall of the stomach and the body/tail of the pancreas. Short gastric vessels are divided using LigaSure to the most cephalad short gastric vessels. The stomach is reflected cephalad, exposing the pancreas. The plane between the posterior stomach and the anterior surface of the pancreas is easy to develop. A laparoscopic intraoperative ultrasound probe introduced through the 12-mm trocar is helpful in selected cases for further localization. It is important in this step to place patients in reverse Trendelenburg position to allow gravity to drop the greater omentum. Care must be taken to avoid injury to the right gastroepiploic vessels and to avoid contact with gastric wall ( Fig. 1 ).
The splenic flexure is mobilized by dividing the splenocolic ligament using LigaSure. This exposes the tail of the pancreas and the inferior pole of the spleen. The colon is reflected medially and the white line of Toldt is divided to develop adequate posterior plane for mobilization of the pancreas in the retroperitoneum. This plane is avascular separating the mesocolon form the Gerota fascia. Splenic attachments to the diaphragm should not be divided to prevent the spleen from flopping in the operative field.
Pancreatic Mobilization
The inferior border of the pancreas is dissected out and a window is created below the pancreatic edge developing a vascular posterior plane between the pancreas and the retroperitoneum. The splenic vein small branches are divided using LigaSure until the posterior surface of the pancreas is fully visualized. The dissection is carried from lateral to medial past the target lesion. The inferior mesenteric vein is identified along the inferior edge of the pancreas. Care must be taken in this step to avoid injury to the retroperitoneal structures, such as the kidney and adrenal gland. Surgeons should maintain a horizontal place during dissection (for subtotal resections, the specimen includes neck, body, tail, and the plane developed behind the pancreas is at the level of the neck).
Pancreatic Transection and Division of the Splenic Vein and Artery
After confirming the site of division, the pancreas is retracted superiorly and anteriorly and the dissection is continued caudal to cephalad exposing the splenic vein. A finger-type dissection may be useful.
The splenic artery is clearly identified and dissected along the superior border of the pancreas. The splenic vein is exposed again posterior to the artery. Splenic artery and vein may be divided en bloc with pancreatic parenchyma unless the pancreatic parenchyma is close to the celiac trunk, in which case the artery is better divided separately.
An articulated rotational endoscopic linear stapler with various thicknesses (staple height, 3.5–4.2 mm) is used to divide the pancreas. In selected cases, the authors apply small titanium clips or fibrin glue (Tisseel, Baxter, Deerfield, Illinois) along the staple line to control bleeding. It is not necessary to completely transect the pancreas with 1 staple firing ( Fig. 2 ). In selected cases, a partial resection facilitates better exposure of the superior edge and the splenic artery for later division. The splenic artery and vein should be clearly identified and divided with linear stapler, taking care not to compromise arteries originating from the celiac trunk. The authors usually use a 45-mm or 60-mm linear stapler, which is placed through the left-sided midclavicular port to allow a direct approach to the pancreas ( Fig. 3 ). In selected cases, the authors transect the splenic artery and vein with the linear stapler before the parenchymal transection. If the splenic vessels were transected proximally, they are transected again at the hilum. An extended lymphadenectomy of the celiac trunk is performed if needed by dividing the attachment of the superior edge of the pancreas to the celiac trunk. Particular care is taken to identify and individually ligate the main pancreatic duct (ultrasound may be useful in selected cases). Once the pancreas and splenic vessels are completely divided, the dissection is continued along the superior edge of the spleen medial to lateral by retracting the pancreas inferiorly to expose any attachment to the retroperitoneum. In a posterior radical antegrade modular pancreatosplenectomy, the left adrenal gland and Gerota fascia are readily exposed. The spleen is freed of its posterior, lateral, and superior attachments. Some surgeons prefer lateral to medial dissection; pancreatic dissection starts laterally by retracting the tail of the pancreas anteriorly and medially, dividing splenic vessels branches to the pancreas. Dissection is continued medially toward the point of transection. Hand-assisted LDP may bear some advantages in selected cases involving dense adhesions, obesity, and large tumors.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree