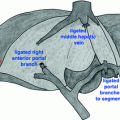
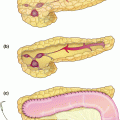
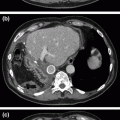
Fig. 24.1
Hypo-enhancing mass (marked by an arrow) along the caudal aspect of the pancreatic body/tail junction, suspicious for adenocarcinoma
Operative Technique for Laparoscopic Distal Pancreatectomy
The patient is positioned in a lazy right lateral decubitus position on a bean bag and secured well to the operating table with straps, tape, and appropriate padding. Usually the right arm is placed on an arm board with an axillary roll, and the left arm crosses the chest and is supported on another arm board. The left knee is flexed, with padding between the legs. This lateral positioning helps with visualization of the body and tail of pancreas, as the stomach and spleen are retracted inferiorly.
The abdominal cavity is accessed with a 5 mm port in the left upper quadrant using the optiview technique with a 5 mm 0° laparoscope. Alternatively, a 10-mm 30° laparoscope is placed above the umbilicus using the open Hasson technique. A diagnostic laparoscopy is then performed for two purposes—first for assessment of any inadvertent intra-abdominal injury during entry into the peritoneal cavity, and second to determine presence of any metastatic disease. If there is any suspicion for any metastatic disease, tissue biopsies are sent for frozen section. If positive for metastases, surgery is aborted, and the patient is referred for definitive chemotherapy.
In the absence of metastatic disease, additional ports are then placed for surgery (Fig. 24.2). These include a periumbilical 10–12 mm port (if a 5 mm port in the left upper quadrant was used initially) and two additional 5 mm ports in the left upper quadrant. These ports are placed in a transverse line along the left abdomen, with the lateral port being in the anterior axillary line. The ports are positioned about 5–8 cm apart in a craniocaudal manner, to permit bimanual operation without any restrictions.
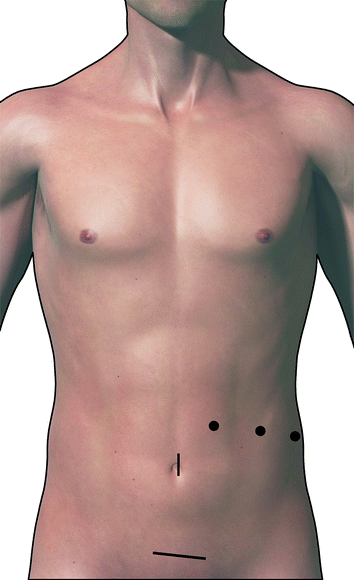

Fig. 24.2
Location of the port sites and Pfannestiel incision for specimen retrieval
Next the omentum is reflected superiorly, and a transverse incision is made in the gastrocolic ligament using harmonic scalpel or bipolar dissector to gain access to the lesser sac. Once in the lesser sac, short gastric vessels are sealed and divided along the greater curvature of the stomach all the way up to the hiatus, keeping the gastroepiploic intact. The patient can be placed in a reverse Trendelenburg position to assist in exposure and visualization of key structures. The transverse colon and splenic flexure are mobilized to gain access to the inferior border of the pancreas. The pancreas is inspected to identify the mass, and intraoperative ultrasound is typically used to assess both the tumor margins and relationship with vascular structures.
Dissection and vascular ligation is then carried out using a stepwise right-to-left or medial-to-lateral approach, recognizing the proximity of the junction of the fourth part of duodenum and jejunum in this area. The left gastric artery can be followed down to the celiac axis, defining the junction of splenic and hepatic arteries. If the patient is placed in Trendelenburg position, the splenic artery will be positioned more anteriorly and the hepatic artery will be angled away [2].
The splenic artery is then dissected at the upper border of the pancreas. Once dissected free, it is encircled using a right-angle clamp and a silicon loop which is secured with a clip, allowing for control in case of bleeding. The artery is then divided and the ends are either divided with a vascular stapler, tied and sutured, or tied and clipped. Ligation of the splenic artery allows for additional mobility of the junction of the pancreatic neck and body and helps to identify the splenic vein insertion into the portal vein. Peritoneum along the inferior margin of the pancreatic body and tail is divided. A retro-pancreatic tunnel can be created under direct visualization. The splenic vein can be transected alone or en-bloc with the pancreatic parenchyma, taking special caution that the main portal vein is away from the plane where en-bloc transection is being performed. Separate transection of these structures ensures safe ligation of the splenic vein without injuring portal or mesenteric veins, and is therefore preferred. Ligation is usually performed using a linear vascular stapler. Following this, the pancreatic parenchyma is transected with a stapler (Fig. 24.3). This is usually done with a vascular load and a slow close technique, closing the stapler over a few minutes followed by slow transection, in attempts to avoid a leak from the cut end of the pancreas [3]. If the pancreatic duct is obviously visible, it is clipped or suture ligated. Another option is cautery transection of gland, suture ligation of the pancreatic duct, followed by oversewing of the pancreatic stump.
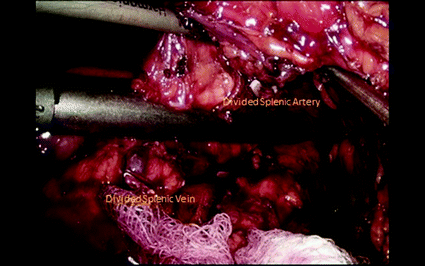

Fig. 24.3
Transection of the pancreas with a stapler, with the divided splenic artery and vein
The remainder of the mobilization is performed from right to left along the retroperitoneum, starting with the fascia over the adrenal and then Gerota’s fascia. Attachments of the spleen to the diaphragm and kidney are taken down, and this tissue is swept with the specimen. Once the specimen is free, it is placed in a retrieval bag and is removed via a small Pfannestiel incision. The specimen should be examined to confirm that the mass has been completely removed. A frozen section at the margin can be obtained, at the discretion of the surgeon. A drain may be left in the pancreatic resection bed. Ports are removed under direct visualization. Fascia for the 10–12 mm port site and Pfannestiel incision is closed. All incision sites are copiously irrigated, followed by skin closure.
Clinical Pearls
Placing the patient in reverse Trendelenburg position after port placement aids in visualization of the key structures.
Intraoperative ultrasound is a valuable tool to assess the tumor margins and its relationship with vascular structures.
Caution must be taken when medial-to-lateral dissection is carried out, given the proximity of the junction of the 4th portion of the duodenum and early jejunum.
Due to the patient positioning, the splenic artery may seem to be positioned more anteriorly (even though it is heading to the patient’s left).
A vascular cartridge for a laparoscopic stapler should be used for pancreatic parenchymal transection and the stapler should be fired using the slow close technique. If the pancreatic duct is seen it should be oversewn with a 3–0 silk suture on a tapered GI needle.
There remains debate regarding intraoperative drain placement after pancreatic resection. Multiple studies have shown that placement of closed suction drains during pancreaticoduodenectomy does not appear to decrease the rate of secondary drainage procedures or surgical exploration and, in fact, may be associated with increased pancreatic fistula (PF) formation and overall morbidity [4–7]. One randomized, controlled trial demonstrated that drains diminish the rate and severity of pancreatic fistula in patients with moderate/high risk for PF, but this could possibly be avoided in the roughly one-third of patients with negligible/low risk [8].
Alternative Techniques
RAMPS is an aggressive surgical approach designed to improve oncologic resection with a higher likelihood of negative (tangential) margins, increased rates of microscopically negative resections, and an improved lymph node dissection. It was originally described as an open technique in 2003 and then later adapted to laparoscopic and robotic surgery. Although it may be associated with improved disease-specific survival, it has similar 5-year overall survival compared to pancreaticoduodenectomy for adenocarcinoma [1, 9].
Alternative techniques include a laparoscopic hand-assist distal pancreatectomy [10, 11], or distal pancreatectomy with splenic preservation [11, 12]. Hand-assist involves a hand port that allows the surgeon’s hand to access the peritoneal cavity during surgery. This assists the surgeon to palpate the tumor, allows for manual retraction and dissection, and application of direct pressure in case there is bleeding. This technique is usually employed in more difficult cases that involve resection of larger tumors, tumors with substantial inflammatory reaction around them, or in obese patients with thick abdominal walls [13].
Distal pancreatectomy with splenic preservation can be performed using what has been described as the Warshaw technique [11] or Kimura [12]. This involves either preservation of the splenic vasculature (Kimura) or preservation of the short gastrics to supply spleen (Warshaw). However, for malignant disease, splenic preservation at the expense of resection margins or thorough lymph node evaluation is not recommended.
Alternative Approaches
RAMPS is an aggressive surgical approach designed to improve oncologic resection with a higher likelihood of negative (tangential) margins, increased rates of microscopically negative resections, and an improved lymph node dissection.
Alternative techniques include a laparoscopic hand-assist distal pancreatectomy or distal pancreatectomy with splenic preservation. For malignant disease, splenic preservation at the expense of resection margins and adequate nodal harvest is not recommended.
Intraoperative drain placement after pancreatic resection remains a great topic of debate. Closed suction drainage has not been shown to decrease the rate of secondary drainage procedures or surgical exploration, and may be associated with increased pancreatic fistula formation and overall morbidity.
Preoperative Evaluation for Pancreatic Adenocarcinoma
The two main goals of preoperative evaluation are to verify the histopathological diagnosis of pancreatic cancer and to determine resectability. This usually involves imaging with any preferred modality, EUS/EUS-guided biopsy, and serum tumor markers. Multiple imaging modalities are available for the evaluation of suspected pancreatic cancer, most common being multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI). Usually the choice of either of these studies depends on available local expertise and the clinician’s comfort with one or the other imaging technique, as there is not an evidence-based difference between the two techniques [14, 15]. In some cases, endoscopic decompression with biliary stent placement may be needed to manage obstructive jaundice (particularly for ampullary masses). EUS is a valuable tool for diagnosis. It has a negative predictive value as high as almost 100% in some series [16, 17]. EUS-guided biopsies help obtain a tissue diagnosis of primary tumor and any suspicious lymph nodes. These biopsies have been reported to have a high sensitivity (85%) and specificity (98%) for malignancy [18], although the utility may be limited in pancreatic body tumors. Serum CA 19-9 is a serum biomarker for pancreatic cancer, and has been shown to aid in diagnosis and can be used as a prognostic marker [19].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree