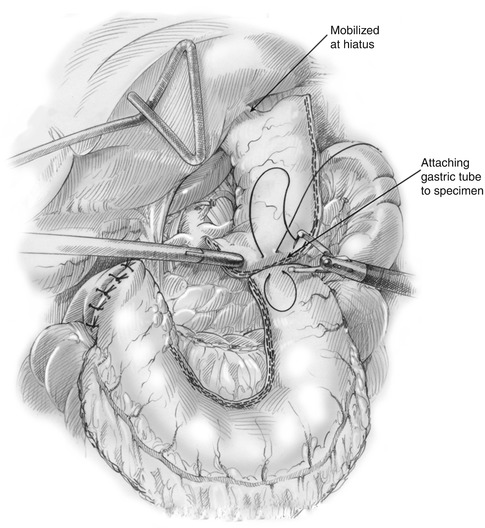
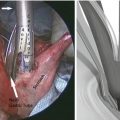
Fig. 11.1
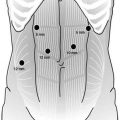
Laparoscopic port placement. The 10 mm port in the right paramedian position is placed first via a direct cutdown technique. The left paramedian port may be converted from a 5 mm port (as shown) to a 10 mm port if a larger camera is desired (© Heart, Lung and Esophageal Surgery Institute University of Pittsburgh Medical Center)
The camera is placed in the left paramedian port position for the majority of the laparoscopic phase. The surgeon works from the right side of the table using the right paramedian and subcostal ports. From the left, the assistant controls the camera and a second grasper for retraction (through the left subcostal port).
Gastric Mobilization
Inspection of the abdomen is performed to evaluate injuries occurring during port placement and to check for liver, omental, or other intraperitoneal metastasis. Biopsies of suspicious lesions are sent for frozen section evaluation. The gastrohepatic ligament is opened and the left gastric vascular pedicle identified. A complete lymph node dissection is performed by dissection of the left gastric and celiac lymph nodes toward the specimen. This dissection is continued laterally along the splenic artery, the superior border of the pancreas, and superiorly toward the crura along the preaortic plane. If the nodes appear bulky or otherwise malignant, they are sent for frozen section evaluation. Once assured of the resectability of the tumor and nodal disease, the crural dissection and complete mobilization of the lower esophagus are performed. The right crus is dissected first; this dissection is continued anterior by transecting the phrenoesophageal ligaments to expose the anterior hiatus. The left crus is often exposed either by a combination of the anterior dissection along the medial crural border and by mobilizing the fundus of the stomach. This combined dissection exposes the retroesophageal window which ensures complete mobilization of the superior portion of the lesser curve completing 360° exposure of the gastroesophageal junction. During this dissection, the exposure is maximized by dividing the left gastric artery and vein with endovascular GIA stapler.
After identifying the gastrocolic omentum, the antrum of the stomach is retracted, and a window is created in the greater omentum, allowing access to the lesser sac. The remaining short gastric vessels are divided while ensuring the dissection is above the gastroepiploic arcade. The fundus is retracted to the right to dissect the remaining retro-gastric short gastric arteries while exposing the left gastric artery and vein. Care should be taken to ensure that all nodes are swept toward the specimen side and to avoid narrowing of the splenic or hepatic arteries. This dissection should complete the mobilization of the fundus and proximal stomach. Gastric mobilization is carried inferiorly to the pyloro-antral region. Meticulous attention must be paid during this phase of the dissection to avoid injury to the gastroepiploic arcade. Mistakenly transecting the arcade often renders the conduit unusable. Direct handling and instrumentation of the conduit portion of the stomach should be avoided. Adequate mobilization has been achieved when the pylorus reaches the caudate lobe. Depending on prior operations and adhesions, enough mobility may require a lysis of adhesions and a partial or a complete Kocher maneuver.
Creation of Gastric Tube
The gastric tube is created prior to the pyloroplasty and placement of the feeding jejunostomy tube to allow time for assessment of conduit viability prior to transition to the thoracoscopic phase. The gastric tube follows the arc of the greater curve of the stomach and is based on the right gastroepiploic artery (Fig. 11.2). Prior to creating the gastric conduit, the nasogastric tube, if previously placed, is pulled back to the mid-esophagus. An endovascular stapling technique allows for a controlled creation of the gastric tube conduit. The first staple load is a vascular (gold) load for the adipose tissue and vessels along the lesser curve above the level of the right gastric artery. No stomach is divided with this firing. The remainder of the firings divides the stomach with 45 mm purple loads (Endo GIA Reloads with Tri-staple Technology, Covidien, Mansfield, MA). The course of the greater curvature is precisely followed by applying traction to the fundus and antrum by the assistants and by traction on the specimen side with the surgeon’s left hand. This three-point traction provides a clear view of the location for gastric transection. Starting at the antrum, the staple line is then directed superiorly, toward the fundus, parallel to the line of the short gastric vessels along the greater curvature. A conduit width of 3–4 cm is preferred (Fig. 11.2). An unusually thick antrum may require that one chooses a greater staple height (e.g., the black Endo GIA loads) to get an adequate staple line integrity. The length of the conduit and margin of resected stomach should be assessed and modified if there is concern for extension of the tumor onto the gastric cardia. Sutures may be placed to reinforce the staple line if there is concern about its integrity though usually not necessary.
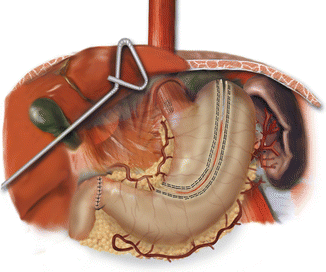

Fig. 11.2
Anatomy of the completed gastric conduit. The right gastroepiploic arcade forms the primary blood supply. The right gastric artery is also preserved and contributes some blood supply to the gastric antrum (© Jennifer Dallal, James D. Luketich, MD)
Pyloroplasty
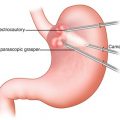
The pylorus is identified and 2-0 Surgidac (Covidien, Mansfield, MA) stay sutures are placed on the superior and inferior aspects using the Endostitch device (US Surgical, Norwalk, CT) (Fig. 11.3). The anterior wall of the pylorus is transected with an ultrasonic shears. The pyloromyotomy is closed transversely in a Heineke-Mikulicz fashion using simple, interrupted 2.0 Surgidac sutures. An omental patch is placed over the pyloroplasty and sutured in place.
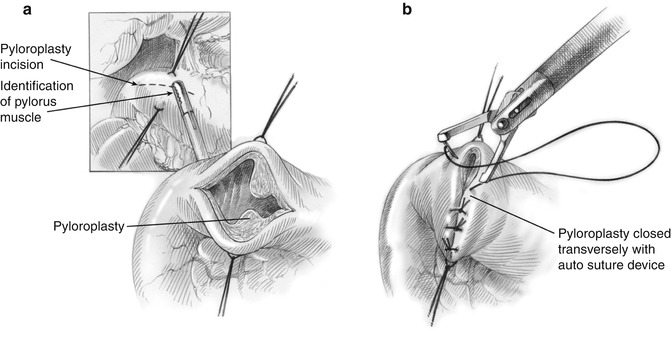

Fig. 11.3
Laparoscopic creation of a pyloroplasty (a) with vertical closure in a Heineke-Mikulicz fashion (b) (© Heart, Lung and Esophageal Surgery Institute University of Pittsburgh Medical Center)
Feeding Jejunostomy Tube Placement
A 12 Fr jejunostomy catheter is placed in the left lower quadrant using a percutaneous technique. The transverse colon is retracted superiorly to expose the ligament of Treitz, and a position on the jejunum 30–40 cm downstream is identified. The antimesenteric border of the bowel is sutured to the abdominal wall with a 2-0 Surgidac suture. The 12 mm right paraumbilical port is used with the camera positioned in the right paramedian location. A Seldinger technique is used to introduce the catheter into the jejunum under direct laparoscopic vision. Air insufflation is used to verify luminal placement. The jejunum is tacked circumferentially to the abdominal wall. An additional suture is placed in the distal limb of the jejunum to prevent volvulus and obstruction.
Preparation for Thoracoscopic Phase
The gastric conduit is assessed for viability. Once viability of the conduit is assured, the most superior portion of the gastric tube is stitched to the specimen (Fig. 11.4). Maintaining the alignment of the conduit to avoid twisting as the stomach is brought into the chest is imperative. The greater curvature along the short gastric vessels is sutured to the staple line of the proximal gastric remnant. If an omental flap has been created, the distal end is sutured to the conduit tip. If hemostasis of the staple line is needed, clips are applied. The specimen and gastric conduit are placed in the lower mediastinum while preserving the proper orientation. If the hiatal opening is large, the crura are reapproximated with a stitch to prevent delayed thoracic herniation of the distal conduit. This step requires considerable judgment by an experienced surgeon because a tight hiatus may compromise the venous drainage of the conduit. A nasogastric tube (if not previously placed) is placed in the esophagus prior to thoracic positioning.