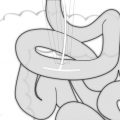
Fig. 14.1
Surgical plan for resection and reconstruction using the right colon to restore esophageal continuity (From Nguyen et al. [7] with permission)
Abdominal Phase
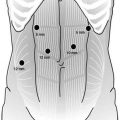
The patient is positioned supine for the initial abdominal phase of the surgery. We employ a standard port placement for most procedures involving the stomach or requiring dissection of the diaphragmatic hiatus (Fig. 14.2). This involves establishing pneumoperitoneum using a Veress needle placed in the left abdomen lateral to the umbilicus at the edge of the rectus abdominis. A 12-mm trocar is placed at this site. We then insert a 5-mm port in the right subcostal region beneath the inferior edge of the liver at the midaxillary line. This port is used for a fixed liver retractor. Another 5-mm port is placed in the right subcostal region at the midclavicular line and a 12-mm port is inserted slightly cephalad and to the right of the umbilicus. These serve as the surgeon’s main operating ports. A final 5-mm trocar is placed in the left upper quadrant and is utilized by the assistant. An initial staging laparoscopy is performed to exclude occult metastatic disease. We frequently do an intraoperative upper endoscopy as well to ensure accurate assessment of the proximal and distal extent of the tumor.
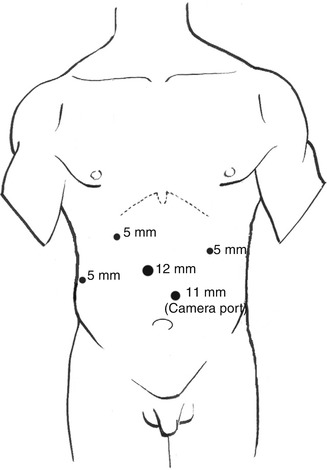

Fig. 14.2
Laparoscopic port placement
After staging laparoscopy excludes the presence of occult metastatic disease, the hepatogastric ligament is divided and the left gastric vessels are exposed. We perform a celiac lymphadenectomy en bloc and then proceed to divide the left gastric artery at the level of the celiac trunk with a single firing of a linear stapler. The stomach is further mobilized by dividing the gastrocolic ligament and short gastric vessels. We routinely perform a partial omentectomy during this phase of the procedure.
After the gastric fundus has been fully dissected, the distal esophagus is mobilized into the mediastinum by opening the phrenoesophageal ligament. We routinely try to obtain at least 6 cm of mediastinal dissection transabdominally. Unlike a gastric pull-up, there is no need to preserve the right gastroepiploic vessels; therefore, these can be divided. The stomach is then transected using a linear cutting stapler. In this situation, it is our preference to leave a small remnant stomach rather than dividing distal to the pylorus as there is a lower risk of anastomotic disruption with a gastrocolic anastomosis compared to a duodenocolic anastomosis. In all cases, we routinely send a frozen section to ensure that a microscopically negative distal margin has been achieved.
At this point, the right colon is mobilized along the white line of Toldt toward the hepatic flexure. We routinely take down both flexures and the transverse colon to minimize tension on the esophagocolonic anastomosis in the chest. Since the blood supply for the colonic interponat is based on the middle colic vessels, it is critical to identify them early and ensure that they are carefully preserved. The ileocolic and right colic vessels are divided at the takeoff of the right colic with a linear stapler. The right colonic mesentery can usually be divided with bipolar cautery. The distal aspect of the colon is divided proximal to the splenic flexure. The proximal aspect of the divided terminal ileum is anastomosed to the colonic splenic flexure. Our preference is to construct a stapled side-to-side anastomosis using a 60-mm linear stapler. The remaining enterotomy is closed with a two-layer running suture using the Endo StitchTM (Covidien, CT). The mesenteric defect should also be closed to avoid potential internal herniation postoperatively. The second anastomosis constructed is the gastrocolic anastomosis. The distal aspect of the colonic interponat is anastomosed to the prepyloric gastric remnant in a side-to-side fashion using a 60-mm linear stapler. Again it is our preference to close the remaining enterotomy using two-layer running suture.
Once these two anastomoses have been completed, we proceed with dividing the upper stomach or distal esophagus in the mediastinum. The cecal pole is sutured to the stomach or esophageal stump in preparation for a colonic pull-up into the thorax (Fig. 14.3). We routinely place a Penrose drain around the distal esophagus in the mediastinum to aid in identification and retrieval of esophagus once in the chest. Our preference during esophagectomy is to remove the tumor during the thoracic phase of the procedure via a small thoracotomy incision; however, in some instances, a large tumor may be extracted from the abdomen if required. We routinely use a plastic wound protector to protect the wound from direct contact with the tumor. The 12-mm trocar incision close to the midline is best suited for tumor extraction and can be enlarged as needed to permit tumor extraction. Our protocol during any esophagectomy is to place a needle catheter jejunostomy in the proximal jejunum to expedite enteral feeding postoperatively while the patient is kept nil per os.
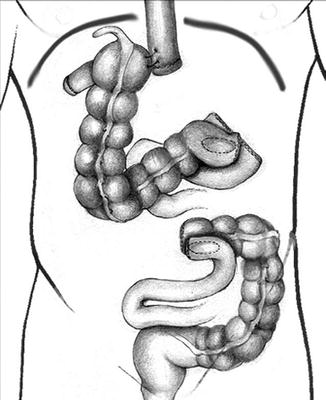

Fig. 14.3
Gastrocolic anastomosis has been performed and the cecum has been anchored to the distal esophagus for pull-up into the chest (From Nguyen et al. [7] with permission)
Thoracic Phase
The patient is then repositioned in left lateral decubitus position under single lung ventilation. Three trocars and a mini-thoracotomy incision (2–3 cm) are placed in the right chest. A plastic wound protector is used in the mini-thoracotomy incision to protect the chest wall from any direct contact with tumor cells. Dissection is initiated by mobilizing the inferior pulmonary ligament. The mediastinal pleura overlying the distal esophagus is incised using bipolar electrocautery, and the Penrose drain encircling the distal esophagus is retrieved. We have found that the Penrose drain greatly assists with esophageal retraction during intrathoracic esophageal mobilization. The esophagus is mobilized up to the level of the azygous vein. We routinely reevaluate the proximal extent of the tumor endoscopically at this point. If the esophagus requires additional proximal mobilization, the azygous vein can be divided with a 60-mm linear stapler. The esophagus is then divided proximally using ultrasonic shears. The remaining distal esophagus and attached colonic interponat can then be pulled into the right chest and separated. The surgical specimen is removed from the chest through the mini-thoracotomy incision.
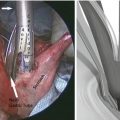
When we initially reported our technique of minimally invasive Ivor Lewis esophagectomy with colonic interposition, we used a circular stapled technique (Video 14.1). A 25-mm anvil was inserted into the esophageal stump and secured in place with a purse-string suture. The ileocecal valve was then dilated to permit passage of the 25-mm circular stapler. The stapler was inserted transthoracically through the 2.5-cm trocar site and positioned through the terminal ileum into the sidewall of the cecum. The esophagocolic anastomosis is then created by firing the circular stapler (Fig. 14.4). The anastomosis was reinforced with a second layer of Lembert sutures. The remaining enterotomy at the terminal ileum was closed using a linear stapler and similarly oversewn with a second layer of Lembert sutures. During our experience with minimally invasive esophagectomy with gastric pull-up, we subsequently changed our practice to construct an entirely hand-sewn thoracoscopically performed esophagogastric anastomosis. The esophagocolic anastomosis can also be constructed thoracoscopically using the Endo StitchTM. Our preferred technique is a double-layer closure with interrupted SurgidacTM sutures (Covidien, CT). We routinely position a nasogastric tube distal to the proximal anastomosis. We place an apically oriented 28-French chest tube as well as a basally oriented 10-French Blake drain (Johnson & Johnson Gateway, Livingston, UK) for postoperative drainage.
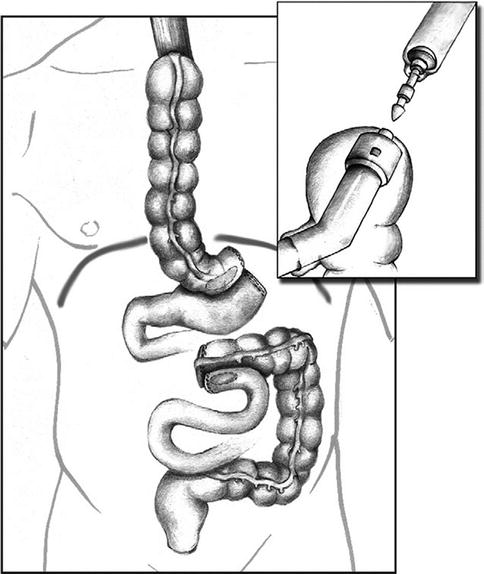

Fig. 14.4
A circular stapled or hand-sewn esophagocolonic anastomosis is created in the chest. The three anastomosis are now completed: (1) ileocolic anastomosis, (2) gastrocolic anastomosis, and (3) esophagocolic anastomosis (From Nguyen et al. [7] with permission)
Postoperative Care
Following esophageal resection, our patients are routinely sent to the intensive care unit for the initial 24–48 h postoperative period. Patients are given patient-controlled analgesia with an opioid infusion. Enteral feeds are commenced via jejunostomy tube at 48 h and advanced toward goal caloric intake as the patient tolerates. The nasogastric tube is left to gravity drainage and suctioned twice a day by the surgical team during inpatient rounds. We routinely perform a water-soluble contrast study on postoperative day 5 to assess for anastomotic leakage (Fig. 14.5). Patients are typically discharged from the hospital by postoperative day 7 provided there is no clinical evidence of leak. Patients receive enteral feeds postoperatively and are allowed to start clear fluids by the second week after surgery. They are gradually advanced toward a soft diet over 3–4 weeks.
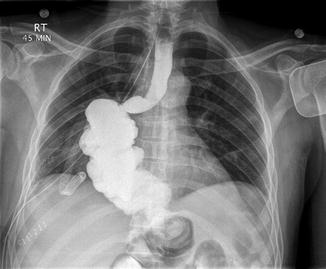

Fig. 14.5
Postoperative water-soluble contrast study demonstrating an intact proximal esophagocolonic and distal gastro-colonic anastomoses without evidence of anastomotic leak (From Nguyen et al. [7] with permission)
Discussion
There is only one published case report to date describing the technique of laparoscopic esophagectomy with colonic interposition [7]. In this paper, the patient had a large esophagogastric cancer involving the gastric body. He underwent laparoscopic/thoracoscopic esophagectomy with interposition of a proximal colonic segment based on the middle colic vessels. Total operative time was 4 h for the laparoscopic portion and 2 h for the thoracoscopic portion. Lymphadenectomy yielded 18 nodes, and microscopically negative proximal and distal margins were achieved at the time of resection. The patient tolerated the procedure well and did not have any evidence of radiologic leakage postoperatively.
Isolauri and colleagues have published the largest series of open esophagectomy with reconstruction by colonic interposition [8]. In that series of 248 patients, they describe an overall mortality of 16 %. Graft necrosis occurred in 3 % of patients and they reported an anastomotic leak rate of 4 %. The major source of morbidity postoperatively in their series was pulmonary complications, which occurred in 8 % of patients. Other published series of open colonic interposition are shown in Table 14.1. These series demonstrate a large variation in overall mortality between 5 and 16 %. Anastomotic leak was noted to occur in 0–14.8 % of patients. The major source of postoperative morbidity in most published series of open esophagectomy with colonic interposition was pulmonary complications. A minimally invasive approach to esophageal resection appears to lessen the risk of postoperative pulmonary complications in high-volume centers. This may be associated with a reduction in overall mortality [21]. The recently published TIME trial that compared minimally invasive esophagectomy with traditional open esophagectomy demonstrated a major reduction in pulmonary complications between the open (29 %) and minimally invasive groups (9 %) [22]. Patients who underwent minimally invasive esophagectomy also had a shorter length of hospital stay and improved quality of life postoperatively compared to those patients undergoing traditional open esophagectomy. It is likely that the advantages observed during laparoscopic esophagectomy with gastric pull-up are transferrable to minimally invasive colonic interposition, although more published series of this technique are needed.
Table 14.1




Selected series demonstrating morbidity and mortality associated with esophagectomy with colonic interposition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree