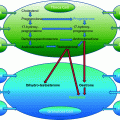
Fig. 25.1
Immature oocyte with compacting cumulus cells surrounding the oocyte
In Vitro Maturation of Immature Oocytes
The source of immature is the most critical point for oocyte maturation, but the culture conditions profoundly affect oocyte maturation in vitro. Although numerous data have been accumulated from the studies, the current rationale for choosing a specific medium for IVM of human immature oocytes appears to stem largely from adapting methods developed from culturing other cell types. Complex culture media, such as TCM-199 medium, Ham’s-F10 medium, and Chang’s medium buffered with bicarbonate and/or HEPES supplemented with various sera, gonadotropins (FSH and LH), growth factors, and steroids, have been most widely used in research and in the clinical application of oocyte IVM [12, 13]. All existing media for oocyte maturation in vitro are the base of complex culture media supplemented with different substances.
Energy Sources
Different energy substrates can greatly influence oocyte meiotic and cytoplasmic maturation [14, 15]. Lactate, pyruvate, and glucose are the main substrates for energy metabolism in oocytes and embryos. Glutamine can also serve as an energy substrate to improve in vitro nuclear maturation of hamster [16] and rabbit [17] oocytes. Lactate is reversibly converted to pyruvate by lactate dehydrogenase. Pyruvate or oxaloacetate, but not glucose, lactate, or phosphoenolpyruvate, supports the maturation of denuded mouse oocytes through meiosis to M-II [18] Synthesis of pyruvate in cumulus cells from glucose provides evidence that these cells are able to influence the nutritional environment of the maturing oocytes [19].
Oocyte utilization of pyruvate is closely dependent upon cumulus cells that can convert lactate or glucose into pyruvate to be used by oocytes [20]. Pyruvate directly affects nuclear maturation in mouse oocytes [21]. It has been confirmed that mitochondrial oxidative metabolism is much more important than anaerobic glucose metabolism for energy production in the mammalian oocytes [22]. However, sodium pyruvate in non-serum maturation medium supports and promotes nuclear maturation of bovine cumulus-denuded oocytes [23]. It has been reported that pyruvate alone is insufficient for oocyte cytoplasmic maturation [24].
The concentration of pyruvate and lactate in the culture medium for embryo development is 0.25 and 30 mM, respectively [25, 26], in which pyruvate concentration is similar to that found in blood and that lactate is much higher, because high concentrations of lactate were found in rabbit oviductal fluid, and this concentration was increased during the first 3 days of ovulation [27]. Most IVM media adopted the concentration of pyruvate and lactate for embryonic development. A deleterious effect of high lactate concentration on early embryonic development has been reported in mouse [28]. Pyruvate alone or pyruvate with lactate can support preimplantation development of mouse embryos until morula stage, but they cannot support the transition to blastocysts without the addition of glucose [29]. Glucose is necessary for embryonic development from morula to blastocyst [30].
Metabolism of glucose through the Embden–Meyerhof pathway is important during bovine oocyte maturation in vitro [31]. The expression pathway of glycolytic metabolism reflects the presence of different mechanisms involved in gene expression/regulation at the transcriptional and translational levels and their accumulation during human oocyte maturation [32]. In mice, glucose treatment of cumulus–oocyte complexes produced elevated cAMP concentrations, which are associated with a decreased incidence of GVBD in hypoxanthine-supplemented medium [33, 34]. Although it has been indicated that glucose may have an inhibitory effect on cumulus-free human oocyte maturation during culture in vitro [35], other results indicated that oocyte maturation medium with glucose is beneficial to bovine and human oocyte nuclear and cytoplasmic maturation in vitro [13, 15]. It has been reported that the optimal concentration of glucose in IVM medium for bovine oocyte maturation may be at 1 mg/mL [15].
Nitrogen Sources
Although amino acids support hamster [16], rabbit [17], porcine [36], and bovine [14] oocyte maturation in vitro, amino acid requirements for oocyte maturation in culture are not fully understood. Essential and/or non-essential amino acids are commonly added to oocyte IVM media. In many species, it has been believed that the addition of amino acids to the culture medium is beneficial to oocyte maturation [13, 37, 38]. Essential amino acids supplemented to a simple chemically defined medium is absolutely required for bovine oocyte cytoplasmic maturation to support subsequent embryonic development, and non-essential amino acids with essential amino acids have a synergic effect on bovine oocyte cytoplasmic maturation in vitro [39].
Apart from amino acid use for protein synthesis, they play important roles as osmolytes [40], intracellular buffers [41], heavy metal chelators, and energy sources [37] as well as precursors for versatile physiological regulators, such as nitric oxide and polyamines [42]. It has been shown that the culture medium with amino acids affect glucose metabolism in mouse blastocysts cultured in vitro [43]. Therefore, amino acids may not be only as nitrogen sources for protein synthesis but also provide the possibility to modify the metabolic pathway.
In addition, the concentration of amino acids in complex media for somatic cell culture may not be suitable for oocyte maturation in culture. Although there is no direct evidence to prove this hypothesis, it has been shown that reduced concentration of amino acids in culture medium is beneficial to embryonic development in vitro [44–47].
Vitamins
There is a paucity of information about the effects of vitamins in culture medium on the maturational and developmental competencies of immature oocytes. The addition of water-soluble vitamins, particularly inositol, to the embryo culture medium enhances the hatching of rabbit and hamster blastocysts [48, 49]. Vitamins also affect glucose metabolism in mouse [43] and sheep embryos [50]. It has been reported that the presence of vitamins in the oocyte maturation medium is important for subsequent bovine embryonic development [51]. The designed IVM medium containing the essential vitamins showed a better result in terms of nuclear and cytoplasmic maturation of immature human oocytes compared to TC-199 medium [13]. Interestingly, Naruse et al. [52] reported that low concentration of MEM vitamins in IVM medium during porcine oocyte maturation in vitro improves subsequent embryonic development. However, the optimal concentration of vitamins in the IVM medium for human oocyte maturation is needed to be determined.
Antioxidant
Oxygen concentration of female reproductive organs is lower than atmosphere [37]. Embryos cultured in low oxygen (5–10%) improve the development [53]. The beneficial effect of reduced oxygen tension may be due to the decrease in reactive oxygen species (ROS) within the cells. Excess amounts of ROS are known to be the major causes of developmental arrest and cell death [54]. The potential deleterious effects of ROS can be eliminated by antioxidant enzyme activities, free radical scavengers, and iron-binding proteins [55, 56]. Antioxidant-related components, such as glutathione, taurine, selenium, apotransferrin, cysteine, cysteamine, and β-mercaptoethanol, are added to IVM medium [57]. Serum contains many factors, including those antioxygen-related components. Therefore, it seems important for serum-free IVM medium to be supplemented with those antioxygen-related components.
Proteins
In 1960s, it has been known that a fixed protein source is essential for development of 2-cell-stage embryos to develop to blastocyst stage [25, 26]. Initially, BSA or FCS or fetal bovine serum (FBS) has been the most commonly used protein source in culture media [58, 59]. Serum is considered crucial for oocyte maturation and may also contain factors essential for human oocyte maturation. The important factors in serum for oocyte maturation could be many growth factors. Patient’s own serum supplemented to IVM medium for their own oocyte maturation in vitro may be a good option for IVM treatment in order to obtain high oocyte maturation rate. In addition, human follicular fluid (HFF) or human peritoneal fluid (HPF) has been used as a protein source supplemented to the maturation medium [4, 60].
Different sources of protein supplement may have different responses for oocyte maturation in vitro and subsequent development as well as the result of pregnancy. We have proven that 10% serum can be replaced by 10% synthetic serum substitute in the designed IVM medium, resulting in approximately 80% maturation rate and more than 90% fertilization rate when the cumulus-free GV oocytes were retrieved from stimulated IVF and ICSI cycle [13]. The commercially available IVM medium from some companies already contains human serum albumin (HSA); therefore, it seems that the extra protein source does not need to be added to those IVM media.
Serum contains complex components, including growth factors, amino acids, and others. Serum fractions of different molecular weights may have different effects on oocyte maturation in culture. Although it is not clear which serum fraction will affect oocyte maturation in vitro, it has been indicated that some fractions contain embryo developmental inhibitory function [61]. Therefore, it seems not certain which component of serum plays an important role in IVM medium during oocyte maturation in vitro. In addition, it has been reported that fatty acid-free BSA might be the optimal supplement to IVM medium due to the higher transcript level of growth factor coding genes accompanied by lower transcript level of Hsp-70 compared with serum [62, 63]. Indeed, in the designed IVM medium containing gonadotropins (FSH and LH), serum can be replaced by polyvinylpyrrolidone (PVP), resulting in less oocyte DNA fragmentation when the IVM medium is supplemented with 0.3% PVP as serum replacement [64].
Gonadotropins
Oocyte maturation in vitro is gonadotropin-independent. However, most IVM media do supplement with FSH or LH or a combination of FSH and LH. The effect of gonadotropins and their relative importance for oocyte maturation in vitro and subsequent fertilization and early development is still controversial. Oocyte maturation in vitro is unlikely undergoing the elaborate cascade of endocrine and paracrine molecular signals that occurred during oocyte maturation in vivo [65–68]. Although Gilchrist and Thompson [69] indicated that today’s IVM system is un-physiological, clinical practice resulted in acceptable pregnancy rate with current IVM system supplemented with gonadotropins [70], while the idea to use FSH and LH is based on the physiological role of FSH and LH in oocyte maturation in vivo. Most likely, FSH and LH act to oocyte maturation-mediated cumulus and granulosa cells because it is believed that there are no FSH and LH receptors on oocytes. Nevertheless, it has been reported that mRNA for FSH and LH receptors are present in mouse and human oocytes, zygotes and preimplantation embryos at different stages [71, 72].
Different concentrations of FSH and LH in the IVM medium have been used. The optimal condition for oocyte maturation should be similar to the physiological concentration of gonadotropins in follicular fluid which contains fully mature oocyte [7, 8, 70, 73, 74]. In addition, the exposure of immature oocytes to different ratios of FSH:LH during maturation in vitro may result in different developmental competencies. Exposure of immature human oocytes to a 1:10 ratio of FSH:LH resulted in significantly higher developmental competence, evidenced by the increased development to the blastocyst stage in vitro compared with FSH alone or no gonadotropins [75]. However, an animal model study indicated that the ratio of FSH:LH is not important for oocyte maturation and subsequent embryonic development [76].
With mechanism studies of oocyte maturation, especially with mouse model, indicated that LH may not be necessary to supplement to IVM medium [34, 77, 78]. It has been believed that epidermal growth factor (EGF) receptor is a central nexus in propagating to the LH signal from the granulose cells, through the cumulus cells to the oocyte [79, 80]. This signal transduction pathway may not be the only way to control oocyte maturation. The increased production of pyruvate and lactate from the cumulus and granulosa cells occurs in response to LH [81, 82]. Therefore, the beneficial effect of LH on the oocytes during IVM involves many possible factors that affect oocyte nuclear and cytoplasmic maturation. Furthermore, it is possible that IVM medium supplemented with physiological concentration of gonadotropins (FSH and LH) stimulates steroid secretion from the cumulus and granulose cells to act on the oocyte maturation.
Steroids
Estradiol and progesterone are the mediators of normal mammalian ovarian function. The actions of estradiol and progesterone on oocyte maturation might be mediated rapidly through the non-genomic mechanism via cell membrane proteins as described in Xenopus [83]. Estradiol may be important not only in regulating oocyte maturation, but also involved in subsequent embryonic development [84]. Interestingly, there is evidence to support the hypothesis that concentrations of progesterone in follicular fluid are closely associated with human oocyte maturity [85].
To consider the effect of estradiol in IVM medium consensus appears to be a concentration of 1.0 µg/ml, which is the concentration in the follicular fluid of pre-ovulatory follicles shortly after the LH peak in cattle [86]. The presence of estradiol in IVM medium had no effect on the progression of human oocyte maturation but improves the subsequent fertilization and cleavage rates [84]. Evidence has revealed that Ca2+ release mechanisms are modified during oocyte maturation [87]. When immature oocytes were cultured in vitro, they acquired the capacity to undergo a single large oscillation of intracellular Ca2+ [88]. However, subsequent sustained oscillations were not observed in some immature oocytes, indicating that these oocytes failed to develop a fully competent Ca+2 signaling system during maturation in vitro. Steroids may be involved in the modification of oocytes during maturation mediated by non-genomic effects, which is referred as ‘oocyte membrane maturation.’ Nevertheless, currently less attention was made to ‘oocyte membrane maturation.’
There seems to be a negative effect of progesterone on bovine oocyte cytoplasmic maturation when it was added to IVM medium with gonadotropins (FSH and LH) and estradiol (Chian et al. unpublished data). IVM medium supplemented with physiological concentration of FSH and LH stimulates estradiol and progesterone secretion from the cumulus and granulosa cells [73, 89, 90]. Therefore, it may not be necessary to add steroids particularly to IVM medium in the presence of gonadotropins and the cumulus and granulose cells.
Growth Factors
There are several growth factors in follicular fluid which contains fully matured oocytes. EGF and transforming growth factor beta (TGFβ) and members of the TGFβ superfamily (TGFβ, inhibin, and activin) are involved in the pathway of regulation of oocyte maturation. It is clear that cyclic AMP (cAMP) is synthesized in the oocyte by constitutively active G-protein coupled receptors [91]. High levels of intra-oocyte cAMP keep the oocyte meiotically arrested by suppressing maturation promoting factor (MPF) activity by stimulating cAMP-dependent protein kinase A (PKA). The oocyte possesses a potent type-3 phosphodiesterase (PDE3), an enzyme that degrades cAMP [92]. Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) exist in the oocyte, involving in the oocyte maturation with a species-specific manner [93] All those signaling mediate to EGF receptor and EGF-like peptide cascade in the cumulus and granulosa cells to control oocyte maturation [94].
EGF alone and associated with gonadotropins induces cumulus expansion and promotes nuclear and cytoplasmic maturation of immature oocytes during culture in vitro [95]. The concentrations of EGF in IVM medium are different from different reports, but most likely use 10.0 ng/ml. It seems that EGF action is mediated by the cumulus and granulosa cells, because denuding oocyte maturation was not affected by the presence of EGF in IVM medium [96, 97]. Therefore, the designed IVM medium can be supplemented with EGF directly when cumulus–oocyte complexes were cultured for maturation in vitro [69, 70, 98].
As mentioned above, serum contains many factors, including the growth factors. Therefore, EGF may not be necessarily added to IVM medium when serum was supplemented to IVM medium for oocyte maturation in vitro [8, 70]. Many commercially available IVM media are ready to use, in which serum was not included in the IVM medium. In this case, it is not clear that whether EGF should be added to IVM medium or not, but it seems better to supplement EGF to the IVM medium when serum was not present in the IVM medium. However, it has to be noted that the active half-life of gonadotropins and growth factors were in IVM medium. Therefore, gonadotropins and growth factors should be supplemented to IVM medium just before use.
Practically, the immature COCs (maximum of 10) can be incubated in an Organ Tissue Culture Dish (Falcon, 60 × 15 mm) containing 1 ml commercially available oocyte maturation medium, in which protein source is already included inside, supplemented with a final concentration of 75 mIU/ml FSH and 75 mIU/ml LH at 37 °C in an incubator with an atmosphere of 90% N2, 5% CO2, and 5% O2 and 100% humidity. Oocyte maturation medium should be prepared for equilibration at least two hours before immature oocyte retrieval (practically, it can be made one day before).
Recently, it has been reported that lysophosphatidic acid (LPA) exerts positive effects on the mouse oocyte IVM process [99]. The addition of LPA to the IVM medium, especially at 30 mM, improved oocyte maturation, fertilization, and subsequent blastocyst development, suggesting that treatment with LPA did not affect the rate of apoptosis of the resultant blastocysts, nor did it had a detrimental effect on spindle normality or the reservation of intact mitochondria in the in vitro-matured mouse oocytes. However, there seem no data available for human immature oocytes.
For human infertility treatment with IVM technology, it seems no breakthrough yet by improving the IVM medium itself. Although there are some ‘new’ ideas or approaches proposed with animal models for IVM of immature oocytes and resulted in high blastocyst formation and fetal production [100, 101], there is no report indicating the ideas or approaches that can be applied to human infertility treatment. Maybe, the ideas or approaches are acceptable with animal industry to use cell cycle modulating agents or spindle formation inhibitors during oocyte IVM, but the usage of these agents for clinical application with human oocyte maturation in vitro should be carefully discussed for its safety issue before adaption of the methodology.
The immature COCs are cultured in the IVM medium in the incubator and allowed to begin the maturation process for 24–48 h. Twenty-four hours after maturation in culture, all of the COCs are stripped for the identification of oocyte maturity. COCs will be denuded using a finely drawn glass pipette following one minute of exposure to a commercially available hyaluronidase solution for 1 min. The mature oocytes are then subjected to insemination by either IVF or ICSI after stripping. The remaining immature oocytes (GV and M-I) will continue to mature in culture for another 24 h. Forty-eight hours after oocyte retrieval (or oocyte maturation in culture), the remaining stripped oocytes are re-examined and if any have matured (M-II) at this point, they will be inseminated immediately by either IVF or ICSI.
In Vitro Fertilization of Immature Oocytes
After 24 and 48 h of immature oocyte retrieval (or oocyte maturation in culture), the immature COCs are examined and if any have matured (M-II) at this point, they will be inseminated immediately by either IVF or ICSI. Nevertheless, ICSI is recommended for the insemination of in vitro-matured oocytes, because we believe that this method offers a greater chance of successful fertilization than does IVF.
Semen can be collected and prepared for the insemination on the day of oocyte retrieval if a mature oocyte has been retrieved from the dominant follicle. Otherwise, semen collection and preparation should be performed the day after oocyte retrieval. Although it is preferable to prepare sperm freshly before ICSI, it does not appear problematic to use sperm prepared on the day of immature oocyte retrieval or the day after for the in vitro-matured oocytes of 48 h after maturation in culture, if the sperm sample is kept at room temperature in the capped tightly tube with the motile sperm.
Commercially available ICSI medium and PVP solution can be used to prepare the ICSI dish. It is also appropriate to use oocyte washing medium for the preparation of the ICSI droplets because the pH of the oocyte washing medium is quite stable at room temperature and atmosphere. However, it is important to note that the ICSI dish should be prepared at least one hour before ICSI and kept at 37 °C in the incubator or on warming stage or plate for equilibration. After ICSI, the individual oocyte is transferred into a droplet (10 µL) of commercially available fertilization medium or embryo maintenance medium in a petri dish for culture in the incubator.
Embryo Development from In Vitro-Matured Immature Oocytes
Sixteen to 18 h after ICSI or IVF, fertilization of the in vitro-matured oocytes is checked under a microscope for the appearance of two distinct pronuclei (2PN) and two polar bodies (sometimes, the polar bodies are fragmented). The fertilized zygotes are to be cultured in the droplets (10 µL) of commercially available cleavage medium or embryo maintenance medium under mineral (or paraffin) oil for one or two additional days, depending upon the number and quality of embryos achieved.
If blastocyst culture is requested, the cleaved embryos should be transferred to new droplets (10 µL) in a petri dish containing blastocyst culture medium or the same embryo maintenance medium under mineral (paraffin) oil two days after ICSI or IVF. The cleaved embryos can be cultured until blastocyst stage in this blastocyst culture medium or embryo maintenance medium without changing the medium (until day 5 or 6 after ICSI or IVF).
Embryo Transfer
It seems that most embryo transfer (ET) in IVM treatment can be done on day 2 or day 3 after ICSI or IVF, because no extra benefit is derived from culturing the embryos to blastocyst stage if the available number of embryos is small. In general, ET should be performed on day 2 after ICSI if the number of embryos obtained is ≤3; ET should be performed on day 3 after ICSI if the number of embryos obtained is ≥3. ET with blastocyst should only be considered if a total of more than 3 or 4 good quality 4-cell-stage embryos are achieved on day 2 of embryo assessment after ICSI or IVF.
The scoring of cleavage stage embryos for transfer is very crucial for pregnancy potential. Since the oocytes may not be matured and inseminated at the same time following maturation in culture, the developmental stages of embryos may be variable in the same patient. Therefore, before ET, all embryos for each patient should be pooled and selected for transfer. The final outcome of pregnancy may depend to a great extent on the experience of the embryologist. The cleavage speed of embryos and the morphological marker of each cleaved blastomere are usually used for scoring the embryo quality of in vitro-matured oocytes. It is recommended that a maximum of 2 embryos be transferred into the uterus, based on the quality of embryos or if blastocysts are obtained the number of embryos for transfer should be only one. It is not true that transferring a greater number of poor quality embryos increases pregnancy and implantation rates.
On the day of ET, endometrial thickness should be measured by transvaginal ultrasound scan. At this point, the endometrial thickness should be at least ≥7.0 mm. If the endometrial thickness is <7.0 mm, the embryo should be cryopreserved and transferred in a subsequent cycle.
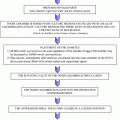
It seems that selecting a good ET catheter is important for establishing successful pregnancy. The ideal ET catheter should be very soft and its tip should be rounded to prevent any trauma to the endometrial lining. In addition, it should be easy to aspirate embryos through the tip under the microscope without air bubbles. Furthermore, the ET catheter should be smooth enough to prevent the embryos from getting stuck to the inner wall of the catheter. Rinsing the inner wall of catheter with a syringe is very important prior to ET. At this point, the oocyte washing medium (2–3 mL) or other commercially available medium contained in a test tube can be used for rinsing the inner wall of the catheter with a syringe. Embryos with transfer medium loading into the ET catheter should not be more than 2–3 µL (Fig. 25.2).
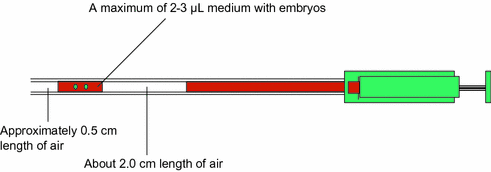

Fig. 25.2
Diagram of embryo loading in ET catheter. Embryo with transfer medium loading into the ET catheter should have maximum 2–3 µL
One of the final key contributory factors to a successful pregnancy is embryo transfer. Careful attention must be paid to both the scientific and clinical aspects of this event. A trial or mock transfer prior to the actual transfer provides very useful information to ensure a curved cervical canal, ascertain the position of the uterus, and any avert foreseeable problems during the actual transfer. It is important that as much mucus as possible is removed from the cervix with a sterile cotton bud before ET. An abdominal ultrasound-guided ET may be recommended in order to confirm that the embryos with the fluid contents of the catheter are in the uterus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree