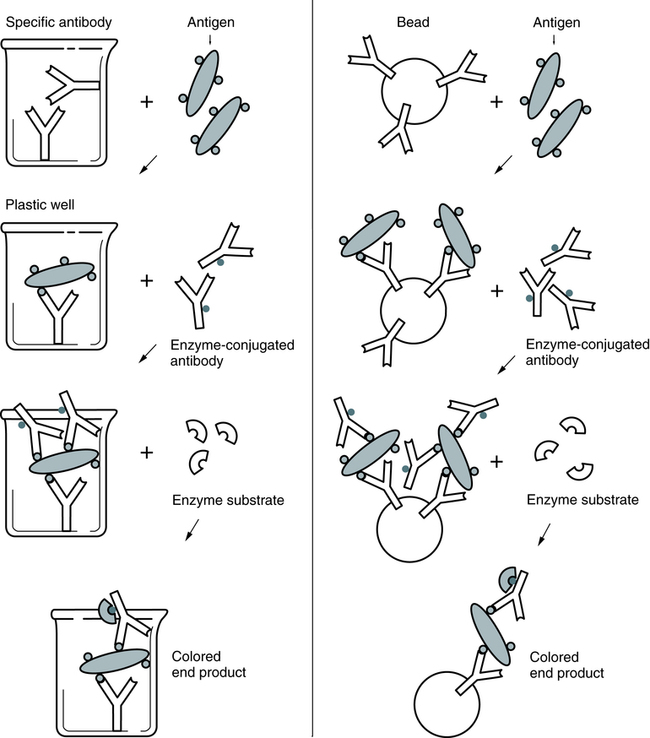
At the conclusion of this chapter, the reader should be able to: • Compare heterogeneous and homogeneous immunoassays. • Name and cite applications of at least three types of labels that can be used in an immunoassay. • Describe and compare chemiluminescence, enzyme immunoassay (EIA), and immunofluorescence techniques. • Briefly compare direct immunofluorescent, inhibition immunofluorescent, and indirect immunofluorescent assays. • Describe the advantages, disadvantages, and application of Q dots, SQUID technology, luminescent oxygen-channeling immunoassay, fluorescent in situ hybridization, signal amplification technology, and magnetic labeling technology. • Analyze a case study related to immunoassay. • Correctly answer case study related multiple choice questions. • Be prepared to participate in a discussion of critical thinking questions. • Describe the principle of the solid-phase immunosorbent assay for pregnancy testing. • Describe the direct fluorescent antibody test for N. gonorrhea. competitive enzyme immunoassay direct fluorescent antibody (DFA) fluorescence in situ hybridization (FISH) fluorescence polarization immunoassay indirect fluorescent assay (IFA) inhibition immunofluorescent assay luminescent oxygen-channeling immunoassay (LOCI) noncompetitive enzyme immunoassay superconducting quantum interference device (SQUID) time-resolved fluoroimmunoassay The principles and applications of enzyme immunoassays, chemiluminescence, and fluorescent substances as labels are presented in this chapter (Table 12-1). Table 12-1 The EIA method uses the catalytic properties of enzymes to detect and quantitate immunologic reactions. An enzyme-labeled antibody or enzyme-labeled antigen conjugate is used in immunologic assays (Box 12-1). The enzyme, with its substrate, detects the presence and quantity of antigen or antibody in a patient specimen. In some tissues, an enzyme-labeled antibody can identify antigenic locations. Various enzymes are used in enzyme immunoassay (Table 12-2). Common enzyme labels are horseradish peroxidase, alkaline phosphatase, glucose-6-phosphate dehydrogenase, and beta-galactosidase. To be used in an EIA, an enzyme must fulfill the following criteria: Table 12-2 Enzymes Used in Enzyme Immunoassays In a representative EIA test, a plastic bead or plastic plate is coated with antigen (e.g., virus; Fig. 12-1). The antigen reacts with antibody in the patient’s serum. The bead or plate is then incubated with an enzyme-labeled antibody conjugate. If antibody is present, the conjugate reacts with the antigen-antibody complex on the bead or plate. The enzyme activity is measured spectrophotometrically after the addition of the specific chromogenic substrate. For example, peroxidase cleaves its substrate, o-dianisidine, causing a color change. In some cases, the test can be read subjectively.
Labeling Techniques in Immunoassay
Types of Labels
Type
Antibody
Comments
Enzyme immunoassay (EIA; enzyme-linked immunosorbent assay, ELISA)
Enzyme-labeled antibody (e.g., horseradish peroxidase)
Competitive ELISA
Noncompetitive (e.g., direct ELISA, indirect ELISA)
Chemiluminescence
Chemiluminescent molecule–labeled antibody (e.g., isoluminol or acridinium ester labels)
Competitive or sandwich immunoassay
Electrochemiluminescence
Electrochemiluminescent molecule–labeled antibody (e.g., ruthenium label)
—
Fluoroimmunoassay
Fluorescent molecule–labeled antigen (e.g., europium or fluorescein label)
Heterogeneous (e.g., time-resolved immunofluoroassay)
Homogeneous (e.g., fluorescence polarization immunoassay)
Enzyme Immunoassay
Enzyme
Source
Acetylcholinesterase
Electrophorous electicus
Alkaline phosphatase
Escherichia coli
β-Galactosidase
Escherichia coli
Glucose oxidase
Aspergillus niger
Glucose-6-phosphate dehydrogenase (G6PD)
Leuconostoc mesenteroides
Lysozyme
Egg white
Malate dehydrogenase
Pig heart
Peroxidase
Horseradish
Oncohema Key
Fastest Oncology & Hematology Insight Engine