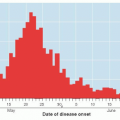
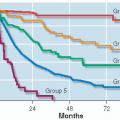
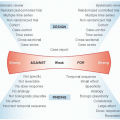
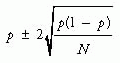Grade of Recommendation |
Clarity of Risk/Benefit |
Quality of Supporting Evidence |
Implications |
1A. Strong recommendation, high-quality evidence |
Benefits clearly outweigh risks and burdens, or vice versa |
Consistent evidence from well-performed randomized controlled trials, or overwhelming evidence in some other form. Further research is unlikely to change confidence in the estimates of benefits and risks |
Strong recommendations apply to most patients in most circumstances without reservation. Clinicians should follow a strong recommendation unless there is a clear and compelling rationale for an alternative approach. |
1B. Strong recommendation, moderate-quality evidence |
Benefits clearly outweigh risks and burdens, or vice versa |
Evidence from randomized controlled trials with important limitations (inconsistent results, methodologic flaws, or imprecision), or very strong evidence of some other research design. Further research (if performed) is likely to change our confidence in the estimates of benefits and risk |
Strong recommendation that applies to most patients. Clinicians should follow a strong recommendation unless there is a clear and compelling rationale for an alternative approach. |
1C. Strong recommendation, lowquality evidence |
Benefits appear to outweigh risk and burdens, or vice versa |
Evidence from observational studies, unsystematic clinical experience, or randomized controlled trials with serious flaws. Any estimate of effect is uncertain. |
Strong recommendation that applies to most patients. Some of the evidence base supporting the recommendation is of low quality. |
2A. Weak recommendation, high-quality evidence |
Benefits closely balanced with risks and burdens |
Consistent evidence from wellperformed randomized controlled trials or overwhelming evidence of some other form. Further research is unlikely to change our confidence in the estimates of benefits and risks. |
Weak recommendation. Best action may differ depending on circumstances or patient or societal values |
2B. Weak recommendation, moderate-quality evidence |
Benefits closely balanced with risks and burdens, with some uncertainty in the estimates of benefits, risks, and burdens |
Evidence from randomized controlled trials with important limitations (inconsistent results, methodologic flaws or imprecision), or very strong evidence from some other research design. Further research (if performed) is likely to change confidence in estimates of benefits and risks. |
Weak recommendation. Alternative approaches likely to be better for some patients under some circumstances. |
2C. Weak recommendation, low-quality evidence |
Uncertainty in the estimates of benefits, risks, and burdens; benefits may be closely balanced with risks and burdens |
Evidence from observational studies, unsystematic clinical experience, or randomized controlled trials with serious flaws. Any estimate of effect is uncertain. |
Very weak recommendation. Other alternatives may be equally reasonable. |
Adapted from Guyatt GH, Oxman AD, Vist GE, et al. for the GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-926. |