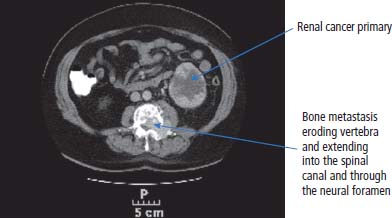
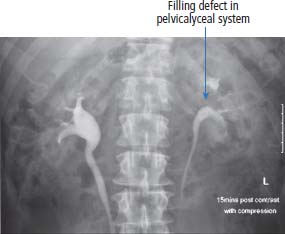
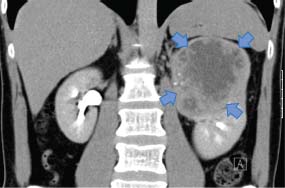
12 In 2010, 10,144 people were diagnosed with kidney cancer and 4189 died of the disease in the United Kingdom. The incidence of kidney cancer is rising faster than almost any other cancer. Around 54% of all kidney cancer patients survive 5 years from diagnosis (Table 12.1). We are at a point where we now understand many of the molecular changes in kidney cancer. Treatments for kidney cancer have been designed targeting these changes and can be given in England and Wales subject to the sometimes draconian decisions and whimsy of the National Institutes for Health and Care Excellence. Over 90% of kidney cancers arise from the renal cortex and are thought to originate chiefly from cells of the proximal convoluted tubules of the nephrons. These tumours are known variously as renal cell cancer, renal adenocarcinoma, clear cell renal cancer, hypernephroma or if you are a really old clinician, Grawitz tumour. About 10% of kidney cancers arise in the renal pelvis and are urothelial transitional cell tumours derived from the transitional cell epithelium of the collecting system. A number of factors have been found to increase the risk of kidney cancer including smoking that also increases the risk of urothelial cancers of the ureters and bladder presumably because of urinary excretion of tobacco-related carcinogens. Obesity and hypertension also increase the risk of kidney cancers. Much recent attention has focused on the molecular biology of kidney cancers including genetic predispositions. The molecular basis of some of the familial syndromes associated with an increased risk of kidney cancer is recapitulated in spontaneous kidney cancer (seeTable 12.2). For example, the mTOR pathway, angiogenesis and the MET receptor have all been found to be disrupted in spontaneous kidney cancers and provide potential therapeutic targets. In clear cell renal cancer, loss of heterozygosity at chromosome 3p (the site of the VHL gene locus) leads to inactivation of hypoxia-inducible factors. This in turn leads to activation of vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR), with resultant new vessel formation and tumour development. VEGFR and EGFR upregulation are features of renal cell cancer that have been exploited for treatment. Table 12.1UK registrations for kidney cancer 2010 Patients with renal cancers commonly present with pain in the loins or blood in the urine. The classic triad of flank pain, haematuria and fever is only found in 10%. Kidney cancers can produce non-metastatic systemic effects including erythropoietin production causing polycythaemia, rennin secretion causing hypertension, parathyroid-hormone-related peptide (PTHrP) production yielding hypercalcaemia and interleukin-6 secretion causing paraneoplastic pyrexia. Less common presentations in males include varicocoeles due to occlusion of the testicular veins. The left testicular vein drains into the left renal vein, whilst the right testicular vein drains directly into the inferior vena cava (IVC). About a quarter of patients present with metastatic disease and the most common sites for secondary spread in kidney cancer are lung, liver, bone and brain. Increasingly kidney cancers are detected as incidental findings during imaging investigations for other indications and this may account in part for the rising incidence in recent years and the improved survival as these tumours are often surgically curable. The Bosniak classification of CT-detected renal cysts predicts the chance that the cyst is malignant based on features such as multi-septation, rim enhancement and presence of soft tissue elements. The urologist will assess the patient in the outpatient clinic, taking a full medical history and examining the patient. Investigations to be organized will include full blood count, liver and renal function tests and a chest X-ray. Further investigation will also include a CT scan of the abdomen (Figure 12.1) and the thorax to define operability. Angiography and an intravenous urogram (IVU) (Figure 12.2) may also have to be performed. Table 12.2 Familial cancer predisposition syndromes and kidney cancers Figure 12.1 Metastatic renal cancer. This CT scan shows a left renal inferior pole mass. In addition, there is erosion of the vertebral body and posterior elements of the third lumbar vertebra. This is associated with extension into the spinal canal causing cauda equina compression and through the neural foramen into the psoas muscle. Kidney cancers are either renal cell cancers (90%) (Figure 12.3) or transitional cell urothelial cancers of the renal pelvis (10%). The main subtypes of renal cell cancer are: clear cell (85%), papillary (10%) and chromophobe (<5%). Clear cell cancers are graded into four Fuhrman categories that are strongly correlated with prognosis. Occasionally primary kidney cancers are oncocytomas that behave relatively benignly and rarely metastasize. In addition, the kidney may be involved by a primary lymphoma or metastases from other sites. The patient with renal cell carcinoma is staged according to the spread of the disease, using the TNM staging criteria. Figure 12.2 An intravenous urogram image demonstrating obstruction of the left pelvicalyceal system at the level of the pelviureteric junction with a filling defect. These appearances were due to a transitional cell carcinoma of the renal pelvis. Transitional cell cancer (TCC) of the renal pelvis arises in the collecting system and may be associated with TCC of the bladder and ureter. The biology, prognosis and treatment are similar to those of bladder cancer. If the patient has no evidence of spread of the disease, then the urological surgeon will arrange for the patient to be admitted for nephrectomy. At operation, the kidney and vascular pedicle and associated lymph nodes are removed, together with the ureter and adrenal. Renal tumours have a propensity to invade along the renal vein. This invasion may extend into the IVC and right atrium. This does not represent a true invasion but is a tumour thrombus. If this is suspected, then a combined approach involving a urologist and a vascular surgeon is advised in an attempt to fully resect the tumour. For smaller and peripheral tumours, nephron-sparing surgery is performed (a fancy way of saying partial nephrectomy). Laparoscopic surgery for smaller tumours is also favoured as the surgical recovery is faster and the blood loss reduced. Figure 12.3 CT scan image of large left upper pole kidney mass. This is a Bosniak 4 renal cystic mass with a large necrotic component, multiple thick seprations and solid enhancing elements. At nephrectomy the pathological diagnosis was renal cell cancer. Locally advanced, inoperable kidney cancer may cause significant symptoms, which may be poorly controlled by systemic palliative measures. These local symptoms can include haematuria, which may be so profound that regular blood transfusion is required, as well as loin pain, which may not respond to opiate analgesia. These symptoms can be treated by radiofrequency ablation, cryoablation, embolization or high-intensity frequency ultrasound. Radical nephrectomy in the presence of metastatic disease may relieve haematuria and pain but has minimal effect on the metastases despite earlier reports, although it may improve response to cytokine therapies. Two trials have shown that patients with metastatic disease who had nephrectomies lived longer. However, the survival benefit is measured in months and has to be weighed against the morbidity associated with surgery. Adjuvant therapy, whether it is radiotherapy to the renal bed, chemotherapy, chemo-immunotherapy, cytokine therapy or anything else, is of no benefit after surgery. Where there are single sites or limited numbers of metastases, there is a surgical option that needs to be considered. The removal of limited numbers of pulmonary metastases, or brain or bone metastases, leads to a chance for cure. Where there are multiple metastases the situation is different. There have been significant changes in the management of metastatic disease as a result of our understanding of the molecular biology of this group of tumours and access to novel therapies. Unfortunately, the rarer kidney cancers such as those associated with fumarate hydratase deficiency do not respond to systemic therapy and for these patients surgery is the only successful treatment option. Chemotherapy is generally ineffective in the treatment of adenocarcinoma of the kidney with response rates below 10%. However, chemotherapy is given in the treatment of urothelial transitional cell tumours that resemble bladder cancers. The response rate of 60–70% is similar to that seen in patients with cancer of the bladder. Unfortunately, these responses are transient and last for a median time of 6–7 months. Until recently, the most important therapy used for metastatic adenocarcinoma of the kidney was immunotherapy. The first agents used were bacillus Calmette–Guerin (BCG) and Corynebacterium parvum, but these have now been replaced by interleukin 2 (IL-2). Initial cytokine therapy for kidney cancer used interferon with modest response rates of 15%. In 1985, the results of treatment with IL-2 were first published, and 60% of patients with kidney cancer were reported to respond to treatment. This high response rate was not confirmed in subsequent studies, which were nevertheless encouraging in that, overall, approximately 20% of patients were seen to respond to treatment. The most significant aspect to IL-2 treatment is that responses are durable. Those few lucky patients who achieve a complete response may be cured of their malignancy. In the original dosage regimen, treatment had significant toxicities and even with current schedules the toxicity is high. It is with some surprise that the authors of this book have observed a resurgence of treatment with interleukin 2. The central role of angiogenesis in the biology of kidney cancer has led to drug treatments that inhibit the VEGF pathway. These include oral small molecule receptor tyrosine kinase inhibitors, “-nibs” and monoclonal antibodies, “-mabs”. The former include sunitinib, sorafenib, pazopanib and axitinib, the latter include bevacizumab. These agents are the most widely used first-line therapies for advanced kidney cancer in the United Kingdom and have been shown to confer an overall survival benefit. In a recent randomized controlled trial pazopanib was compared to sunitinib in metastatic renal cell cancer. Both were equally effective, but pazopanib came out on top in terms of safety and quality of life. The mammalian target of rapamycin (mTOR) pathway is downstream of the phosphoinositide 3 kinase and AKT (known as protein kinase B) pathway that is regulated by the phosphatase and tensin homologue (PTEN) tumour suppressor gene. This provides a valuable target in advanced kidney cancer. Temsirolimus and everolimus are mTOR inhibitors that are used as first-line therapy especially in advanced poor prognosis kidney cancer. The optimal first-line treatment for advanced kidney cancer depends upon the organ function and performance status as well as the risk of progression of the malignancy. Many current trials aim to establish the gold standard first-line therapy as well as the most appropriate sequencing of lines of treatment. The prognosis for localized adenocarcinoma of the kidney is variable. The survival rate for patients with good prognosis tumours is 60–80%, but if there is vascular or capsular invasion, only 40% survive 1 year. The median survival for patients with metastatic disease was 9 months. These statistics have significantly changed as a result of the development of new treatments for kidney cancer; with systemic treatment the median survival for patients with metastatic disease has been extended to 2 years. Kidney cancer survival is significantly longer for those who live in the least deprived areas of the United Kingdom compared to those who live in areas of greater material deprivation. Overall, 10% of patients with metastatic renal cell cancer survive 5 years from diagnosis and this group represents a curious feature of the malignancy. Even in the absence of metastases at presentation, the outlook for patients with transitional cell tumours is very poor, with 10% surviving for 1 year and 5% for 2 years. Case Study: A heart of gold and an oncocytoma.
Kidney cancer
Epidemiology
Pathogenesis
Percentage of all cancer registrations
Rank of registrations
Lifetime risk of cancer
Change in ASR (2000–2010)
5-year overall survival
Female
Male
Female
Male
Female
Male
Female
Male
Female
Male
Kidney cancer
2
4
10th
7th
1 in 90
1 in 56
+38%
+27%
55%
53%
Presentation
Outpatient diagnosis
Syndrome
Kidney cancer risk
Other manifestations
Gene affected
von Hippel–Lindau disease (VHL)
70% develop clear cell RCC by age 60 years
Phaeochromocytoma (Type 1 VHL) Haemangioblastoma CNS or retinal (Type 2 VHL)
VHL 3p regulates hypoxia inducible factor 1α (HIF)
Hereditary papillary renal cell cancer (HPRCC)
Type 1 papillary RCC
None
MET (receptor for hepatocyte growth factor)
Hereditary leiomyomatosis renal cell cancer (HLRCC)
10% develop type 2 papillary RCC (10%)
Leiomyomata of skin and uterus
Fumerate hydratase (FH)
Birt–Hogg–Dubé syndrome (BHD)
15–30% develop RCC
Skin fibrofolliculomas, lung cysts
Folliculin (FLCN)
Staging and grading
Treatment
Surgery
Management of an inoperable primary tumour
Adjuvant treatment
Management of metastatic kidney cancer
Chemotherapy
Immunotherapy
Anti-angiogenic therapy
Targeted therapy
Prognosis
 ONLINE RESOURCE
ONLINE RESOURCE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


