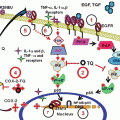
Fig. 1
Molecular targets of genistein on cancer cells. Genistein exhibits either stimulatory or inhibitory effects on different signaling pathways. Abbreviations: COX-2 cyclooxygenase-2, FAK focal adhesion kinase, HIF-1α hypoxia-inducible factor 1α, MAPK mitogen-activated protein kinase, MMPs matrix metalloproteinases, NF-κβ nuclear factor κβ, PTK protein-tyrosine kinase, VEGF vascular endothelial growth factor
3 Animal Studies
Because sexual maturation is similar among species, animal models provide a practical design for the study of variety endocrine functions. Numerous endocrine-mediated functions in mice or rats, for example, are comparable to other mammalian species including humans. Due to these species-related similarities, rodents may provide high reproducibility model for investigation in humans (Russo et al. 1996; Medina 2007). In the years since, there has been considerable investigation of the potential anticancer effects of soy products against breast cancer in animal studies. Soy foods have been suggested to have both positive health effects and potentially adverse effects as a consequence of the content of isoflavones—a natural phytoestrogen with potential hormonal activity due to their similar chemical structure to 17-β-estradiol. In this regard, there are two recent experiments which suggested that soy protein isolate fails to recruit appropriate co-activators at estradiol-inducible genes and behaves like selective estrogen receptor modulator rather than weak estrogen in the developing rat mammary gland (Miousse et al. 2013; Ronis et al. 2012). Long-term administration of isoflavones to rats (24 months) led to substantial increase in gene expression in the mammary gland and thus these natural compounds significantly influence cell signaling (Chalabi et al. 2010). Molzberger et al. (2013) found that isoflavone exposure to female rats during puberty is sufficient to reduce the proliferative response of the adult mammary gland to estradiol but not to reduce the response of progesterone receptor (PR). Importantly, studies focused on the detailed analysis of the soy isoflavones’ estrogenicity in mammary carcinogenesis are still lacking.
There are some scientific approaches investigating antitumor efficacy of soy products or isolated soy isoflavones in breast cancer animal models. Numerous experiments realized in this research were based on the ovariectomized athymic mouse model; on the other hand, it is the chemically induced rat mammary carcinogenesis.
3.1 ER-Positive Breast Cancer Model in Ovariectomized Athymic Mice
A variety of studies have found that soy isoflavones stimulate estrogen receptor-positive (ER+) human breast cancer cell xenoplasts in ovariectomized athymic mice that first raised fear that soy isoflavones might be contraindicated for breast cancer patients. The Helferich’s group demonstrated that the isoflavone, genistein, stimulates the growth of estrogen-dependent human breast cancer (MCF-7) cells in vivo (Hsieh et al. 1998). In this study, MCF-7 cells were implanted s.c. in ovariectomized athymic mice, and the growth of the estrogen-dependent tumors was measured weekly. Tumors were larger in the genistein-treated group than they were in the untreated control group. The same group using the same model investigated whether consumption of genistein from soy protein will have similar effects on estrogen-dependent tumor growth as pure genistein (Allred et al. 2001). Soy protein diets containing varying amounts of genistein increased estrogen-dependent tumor growth in a dose-dependent manner. Similar results with stimulatory effects of isoflavones on ER+ breast cancer cells xenoplasts in ovariectomized mice were confirmed also in other experiments (Ju et al. 2001; Allred et al. 2004). Furthermore, using the same model, dietary genistein negated the inhibitory effect of tamoxifen on MCF-7 tumor growth, lowered estradiol levels in plasma, and increased expression of estradiol-responsive genes (e.g., pS2, progesterone receptor, and cyclin D1) (Ju et al. 2002). The same group evaluated the interaction of dietary genistein and an aromatase inhibitor, letrozole, on the growth of tumors in an aromatase-expressing breast cancer xenograft model (MCF-7Ca) in the presence and absence of the substrate androstenedione. Dietary genistein increased the growth of MCF-7Ca tumors implanted in ovariectomized mice, and in the presence of androstenedione and letrozole, dietary genistein reversed the inhibitory effect of letrozole on MCF-7Ca tumor growth in a dose-dependent manner (Ju et al. 2008).
Other research using athymic mouse model has found that more processed soy products result in faster tumor growth than mice exposed to less processed soy product even if the amount of genistein in both groups was the same (Allred et al. 2004). There are some explanations for this finding. Soy foods’ processing causes greater increase of serum genistein levels in animals. Food processing can remove non-nutritive components of soy products which are able to inhibit the tumor-stimulatory effects of isoflavones. These changes in the diet composition may increase circulating (and probably target tissue) concentrations of genistein, which activates ER-mediated processes required for tumor growth stimulation in the mouse postmenopausal breast cancer model (Allred et al. 2005). Using a similar rodent model of breast cancer, the Thompson’s group also observed stimulatory effects of isolated soy protein on tumor growth (Saarinen et al. 2006).
Daidzein, the second most prominent isoflavone from soy, has been shown to have only modest stimulatory effect on the growth of MCF-7 cells in the athymic ovariectomized mouse model. Daidzein can be further metabolized to equol by bacterial microflora in the rodent intestine. Equol is structurally similar to estrogen and it is a potent ligand for ERβ. Thus, it has a potential to exert a greater estrogenic effect in comparison with the parent molecule—daidzein (Setchell et al. 2005; Setchell and Clerici 2010). Ju et al. (2006) evaluated the estrogenic potential of daidzein and synthetic equol to affect the growth of MCF-7 cells in vitro and in vivo. Similarly, dietary daidzein had a slight but significant stimulatory effect on MCF-7 tumor growth in ovariectomized athymic mice. However, no significant induction of pS2 mRNA (an estrogen-responsive marker) in tumors by dietary daidzein was found. On the other hand, dietary equol treatment did not stimulate MCF-7 tumor growth in this mouse model. No statistical differences in tumor size, proliferation, and pS2 expression among any treatment groups were found. Total daidzein or equol plasma levels in mice fed with the isoflavones were in the range that stimulated in vitro MCF-7 cell growth. The results of Ju et al. (2006) suggested that pharmacokinetic and/or metabolic factors attenuate the estrogenic effects of soy isoflavones in vivo.
There is, however, warrantable debate about the merits of using athymic ovariectomized mouse model of breast cancer to evaluate effects in humans. There are several limitations and weaknesses of abovementioned studies (Messina et al. 2006a; Messina and Wood 2008). A specific criticism is that unlike pre- and postmenopausal women, these mice do not produce sufficient endogenous estrogen to promote development and growth or to even maintain estrogen-dependent tumors. Therefore, the effects of soy isoflavones on MCF-7 cells xenoplasts were evaluated in an estrogen-deficient environment that does not adequately reflect conditions in both pre- and postmenopausal women. It has been debated that estrogenic effect of isoflavones characterized by stimulatory effects on tumor growth may be evident only in this model with hypoestrogenic environment. Nevertheless, there are some rodent studies in which estrogen levels were more reflective of the hormonal status of postmenopausal women and, however, tumor stimulation caused by isoflavonoids was found. It is the mouse model with implanted MCF-7a cells which serve as an autocrine source of estrogen in the ovariectomized, immune-suppressed animals (Ju et al. 2008) and model with silastic implants in mice that yield low circulating plasma estradiol levels similar to those observed in postmenopausal women (Ju et al. 2006). Another critic of abovementioned studies is the use of high oral doses of isoflavonoids. Isoflavones are relatively weak estrogen agonist compared to estradiol, but the markedly higher circulating concentrations of biologically active (unconjugated) genistein in certain strains of mice cast doubt on the value of the use of these rodents for gaining insight into the effects of isoflavones in humans (Setchell et al. 2011). In most of in vivo studies, the doses of isoflavonoids (750 ppm) were at least 15 times higher than the amounts found in traditional Asian diets (30–40 ppm) (Messina et al. 2006b). In addition, serum isoflavonoid molar ratios differ between rodents and humans also due to the rodent intestinal bacteria which effectively convert daidzein to equol, whereas only 30–50 % of humans are carriers of this specific bacterium (Gu et al. 2006). Even in human equol producers, genistein is the predominant serum isoflavone after the ingestion of soy products, whereas equol predominates in most other species including rodents. Importantly, from the clinical point of view, the isoflavone doses required for estrogen-stimulatory effects in women have not been identified yet. Furthermore, the important feature of this model is the lack of immune function that may eliminate possible mechanisms by which soy isoflavones suppress carcinogenesis. Guo et al. (2007) analyzed whether genistein modulation of the immune responses might contribute to the increased host resistances to tumors in adult female B6C3F1 mice. Pretreatment with genistein by gavage enhanced host resistances to the B16F10 tumors and DMBA-induced carcinogenesis. In addition, the exposure of mice to genistein increased the activities of in vivo cytotoxic T lymphocytes and natural killer cells. Another possible limitation of this model may be the extent how existing MCF-7 xenoplasts in nude mice reflect tumors in patients with breast cancer. These model tumors are transformed and consist of cells that are very sensitive to proliferative effects of estrogen or even genistein with weak estrogenic properties. Finally, there are other relevant rodent models (e.g., chemically induced rat mammary carcinogenesis) that have shown the suppression rather than stimulation of mammary tumor growth (Ma et al. 2014; Ronis et al. 2012; Dave et al. 2010).
3.2 Chemically Induced ER-Positive Tumors in Rats
Rat mammary carcinogenesis in female rats induced by 7,12-dimethylbenz[a]anthracene(DMBA) is well-established hormone-dependent breast cancer model which tremendously increased the understanding of chemically induced tumors in the mammary gland. The conversion of normal mammary epithelial cells to adenocarcinomas by DMBA is relevant to the events leading to breast cancer in women. This model is favored by the National Cancer Institute for the evaluation of breast cancer chemopreventive agents (Kelloff 2000). There are numerous recent studies which investigated whether soy isoflavones have any effect on the chemoprevention and suppression of experimental rat mammary gland cancer development induced by DMBA. Ma et al. (2014) found that soy isoflavone intake inhibited the development of DMBA-induced mammary tumors in normal and ovariectomized rats. In this study, mRNA and protein expression of ER was significantly higher in treated groups. Moreover, isoflavone treatment significantly decreased 8-hydroxydeoxyguanosine content and increased superoxide dismutase level in normal rats and decreased malondialdehyde concentrations in ovariectomized rats. In another study of this group, isoflavone or equol intake significantly inhibited the incidence and lengthened the latency period of DMBA-induced mammary tumors in ovariectomized rats probably due to increased antioxidant and estrogenic activities (Ma et al. 2014). In another study, the supplementation of genistin alone or with selenium provided antioxidant defense with high-potential chemopreventive activity against DMBA-induced mammary tumors more than selenium alone. These positive effects of genistein were characterized with decreasing levels of tumorigenicity, endocrine derangement, and oxidative stress in premenopausal breast cancer model (Hamdy et al. 2012).
The status of glycoconjugates (protein-bound hexose, hexosamine, sialic acid, and fucose) in plasma or serum serves as potential biomarkers for assessing tumor progression and therapeutic interventions. Pugalendhi et al. (2011) observed that genistein and daidzein in combination protected the structural integrity of the cell surface and membranes during DMBA-induced rat mammary tumorigenesis. The same combination of isoflavones showed anti-lipid peroxidative efficacy and modulatory effect on phase I and phase II detoxification cascade during DMBA-induced rat mammary carcinogenesis (Pugalendhi and Manoharan 2010). In addition to genistein and daidzein combination in DMBA breast cancer model, Aidoo and Manjanatha (2011) suggested that consuming diets containing more than one soy isoflavones as opposed to taking supplements in isolation could impart some benefits.
There are also recent studies, which evaluated the efficacy of two equol enantiomers S-(−)equol and R-(+)equol dietary administered in animal model of DMBA-induced mammary gland cancer. In the first study, S-(−)equol had no chemopreventive action, nor was it stimulatory. In contrast, R-(+)equol significantly decreased tumor frequency and lengthened tumor latency and tumors were less invasive. Both enantiomers had no effect on absolute uterine weight (Brown et al. 2010a). In the second study, rats were exposed to S-(−)equol or R-(+)equol during the neonatal (0–21 days) or prepubertal (21–35 days) periods only. The exposure of both equol enantiomers resulted in a decrease in immature terminal end structures and an increase in mature lobules. Despite these morphological changes to the mammary gland, neonatal and prepubertal exposure to equol had no long-term chemopreventive effects in DMBA-induced mammary carcinogenesis (Brown et al. 2010b). In addition, significant tumor-suppressive effects of soy isoflavones in hormone-dependent rat mammary carcinoma model induced by ethyl methanesulfonate (Ono et al. 2012) or N-methyl-N-nitrosourea (NMU) (Dave et al. 2010) were found. However, in the experiment with NMU, soy-rich diet may influence the development of more aggressive carcinomas by enhancing progesterone receptor-A-dependent signaling.
In contrast to plentiful above noted results with significant antitumor effects of isoflavones in chemically induced rat breast cancer model, there is the study where postpubertal exposure of Donryu rats to isoflavones at an estrogenic dose resulted in promotion of mammary and uterine carcinogenesis induced by DMBA and N-ethyl-N′-nitro-N-nitrosoguanidine. Authors suggested that this result might be caused by the activation of ER-dependent signaling and alteration of the molecular tumor environment in the target organs (Kakehashi et al. 2012). Possible explanation for this relatively isolated negative result in rat model may be the use of different strain of Donryu rats with different genetic background when compared to often investigated Sprague-Dawley rats. In conclusion, however, findings noted above demonstrated clear chemopreventive and tumor-suppressive effects of soy isoflavones in a well-established chemically induced breast cancer model.
3.3 Effects on ER-Negative Breast Cancer Model
The effects of genistein on hormone-independent breast cancer model in vivo have been less investigated. To evaluate the effect of dietary genistein on tumor growth in vivo, genistein was fed to female athymic mice inoculated with ER-negative MDA-MB-231 cells. After solid tumor masses had formed, mice were fed genistein at a dose of 750 mg/kg. This dose of genistein did not significantly alter tumor development (Santell et al. 2000). Similarly, genistein (at the same dose) fed 3 days before cells were inoculated into mice did not significantly inhibit tumor formation or growth. However, another study reported that lower dose of genistein (250 mg/kg) suppressed the growth of MDA-MB-231 cells implanted into female nude mice orthotopically (Li et al. 2013). Possible explanation of this discrepancy in results may be the orthotopic mouse model used by Li et al. that provides probably better physiological conditions in comparison with subcutaneous implantation of cancer cells as realized by Santell et al. In addition, Li et al. (2013) in the same experiment suggested that soybean genistein can epigenetically restore ERα expression probably through the inhibition of expression and activity of enzymes that regulate chromatin structure in MDA-MB-231 breast cancer cells. These changes in turn increase tamoxifen-dependent anti-estrogen therapeutic sensitivity of MDA-MB-231 cells in vitro and in vivo. ER-negative breast cancers are unresponsive to tamoxifen therapy and more aggressive, resulting in a poorer prognosis. Therefore, novel therapeutic combination approach using bioactive soybean product and tamoxifen therapy in refractory ERα-negative breast cancer might be a good option but needs to be evaluated further (Li et al. 2013).
In preclinical research, the effects of daidzein on ER-negative breast cancer are insufficient and inconsistent. Athymic female mice with ER-negative MDA-MB-435 xenoplasts were treated orally with 10 mg/kg genistein or daidzein. Results showed that daidzein increased while genistein decreased mammary tumor growth. Daidzein increased lung and heart metastases while genistein decreased bone and liver metastases. Combined soy isoflavones did not affect primary tumor growth but increased metastasis to all organs tested (Martínez-Montemayor et al. 2010). Daidzein alone significantly upregulated 9 of 84 genes that regulate proliferation and protein synthesis (e.g., EIF4G1, eIF4E, and survivin). On the other hand, metastatic effects of eqoul on MDA-MB-435 cells were not confirmed in vitro (Magee et al. 2014). In this regard, however, the identity of MDA-MB-435 as breast cancer cells was questioned (Lacroix 2009), and it will be necessary to verify the relevance of this cell line for future breast cancer research.
4 Soy and Breast Cancer: Results from Clinical Research
Soy and soy isoflavones’ consumption has been studied in relation to breast cancer. Decrease in disease incidence was noted likely as a result of their ability to mimic natural estrogens, which are known to play a role in breast cancer progression (Pavese et al. 2010). Wu et al. study (2008) showed 18 % lower breast cancer risk in Asian women with the highest isoflavone intake (≥10.6 mg/day). However, Dong and Qin (2011) described only 11 % decrease in risk of breast cancer incidence. They documented that the risk of breast cancer incidence decreased, on average, by 4 % for every 10 mg/day increase of soy isoflavones intake. No significant effects were found in Western population. The Westerners’ results may largely be attributed to the different amount of soy consumption which is obviously far below even the lower levels of intake in an Asian population (Xie et al. 2013; Dong and Qin 2011). In Asian women, the lowest risk was noticed in the category of the highest of isoflavone intake. The risk in the low- and medium-dose category was similar (Table 1).
Table 1
The doses of isoflavones in humans
Asian women | |
The highest dose category | >25 mg/day |
Medium-dose category | 15–25 mg/day |
The low-dose category | 5–15 mg/day |
Western women | |
High-dose category | >1000 μg/day |
Low-dose category | 500–1000 μg/day |
The fact that increased prevalence of clinical cancer may occur within a single generation after migration from the East to the West points out that cancer is not entirely genetic and that it can be pharmacologically altered by dietary constituents such as soy (Pavese et al. 2010). In Western diet, the majority of isoflavone intake comes from non-soy foods, such as soy additives in baked goods, tuna, or coffee. Asian people’s isoflavone consumption is through soy foods, such as tofu, soy milk, miso, etc., in which the isoflavone content is similar (Xie et al. 2013; Horn-Ross et al. 2000). Early isoflavone exposure can protect against breast cancer as they may exert their putative protective effects by stimulating breast cell differentiation so isoflavone intake since childhood or adolescence may influence the risk of breast cancer incidence in adulthood (Messina and Wu 2009). One of the factors affecting the bioavailability of isoflavones may be frequency of ingestion. Relatively small doses of soy throughout the day can keep an optimum steady-state serum isoflavone level, as opposed to a single dose at once. Also interindividual differences in daidzein metabolism may play important role in the soy and breast cancer association. Isoflavan equol, a bacterially active derived metabolite of daidzein that is produced only by 25 % of Westerners, has been proposed as an especially beneficial compound. Equol binds with greater affinity to both ERs, with a higher binding affinity for ERβ having pro-apoptotic properties, than its parent compound daidzein. Clinical response is usually limited to people who are “equol producers,” and its production varies across individuals and populations, equol-producer status should be considered as it may modify the estrogenic potency of isoflavones (Dong and Qin 2011; Nagata 2010; Setchell et al. 2002; Setchell and Cole 2006; Goodman et al. 2009; Ward et al. 2008). Until now limited clinical research has been done about association between the intake of soy isoflavones and its effect in breast cancer patients. As mentioned above, early life exposure to isoflavone in Asian women has protective effect on breast cancer risk (Lee et al. 2009; Xie et al. 2013) and has been associated with lower Her2/neu and proliferating cell nuclear antigen expression in malignant compared to benign breast tissue (Maskarinec et al. 2009). Clinical research studied mainly focus on determination of association between the intake of soy food and menopausal status, ER status and effect on adjuvant therapy, recurrence and survival among breast cancer patients.
4.1 Menopausal Status and Exposure to Soy Isoflavones
Endogenous estrogen levels in premenopausal women are greatly different from postmenopausal women. Since isoflavones possess estrogen-like effects it was assumed that menopausal status may play a modifying role in the isoflavone intake and breast cancer risk association (Chen et al. 2014; Dong and Qin 2011). Many epidemiological studies explored this association but inconclusive results were obtained. Lee et al. (2009) noted protective effect of isoflavone intake against premenopausal breast cancer, but no significant association between isoflavone intake and postmenopausal breast cancer. Isoflavone consumption did not affect estradiol or estrone, but it reduced FSH and LH in premenopausal women (Hooper et al. 2009). Other epidemiologic analyses suggest that high soy intake is associated with an approximate one-third reduction in the risk of both pre- and postmenopausal breast cancer among Asian women (Messina and Wu 2009). Stronger inverse association of isoflavone consumption with breast cancer incidence in postmenopausal women than in premenopausal women was presented in more studies (Xie et al. 2013; Nagata 2010; Dong and Qin 2011; Wu et al. 2008; Yamamoto et al. 2003).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree