Fig. 14.1
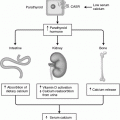
Human islet isolation. a Human islet isolation occurs in a specialized laboratory. b The pancreatic duct is cannulated after the pancreas is trimmed. c The sectioned pancreas is loaded into the Ricordi chamber for digestion. d Isolated islets are stained with dithizone and counted
Density Gradient Purification
It was discovered a number of years ago that islets infused into the portal vein with large amounts of accompanying exocrine tissue can lead to portal vein thrombosis which can be fatal [40]. The pancreatic digest is therefore, purified by density gradient centrifugation, enabling ‘pure’ islets to be retrieved. Islet purification takes advantage of the fact that islets and exocrine tissue have different buoyant densities. When placed in specially developed media of known density and spun on a centrifuge, tissue will migrate to a layer corresponding to their own density, with the less dense islets settling at a higher density than exocrine tissue. The introduction of the COBE 2991 machine by the Leicester Group, originally utilized for blood component separation, has become one of the key factors in achieving sufficient numbers of purified islets for clinical use [41].
Islet Culture
While the islets used in the original Edmonton Protocol were transplanted immediately after isolation, culturing islets post-isolation is believed to be important for their recovery from isolation-induced damage and enables islets to be carefully assessed before infusion. It also enables immunomodulatory therapy to be started in the recipient and potentially reduces the immunogenicity of the graft. A culture period also greatly enhances the practicality of an islet transplant in terms of planning for the patient’s admission and the availability of the radiology suite. However, this may be at the cost of impaired revascularization subsequent to transplant, due to the loss of intra-islet endothelial cells during this culture period. Therefore, essential components of the culture conditions for human islet preparations are sufficient oxygen and nutrient supply. During the culture stage, the maintenance of the tridimensional islet cluster and preventing islet mass loss should be accomplished during the culturing phase. To date, sufficient investigation of optimal culture conditions has occurred, yet in spite of this, protocols have yet to be standardized and culture conditions may vary between islet isolation centers [42]. Other considerations like media composition, seeding density, and incubation temperature play a significant role in maintaining viability and recovery [42].
During the 24–48 h of islet culture, 10–20% of the islet mass is ‘lost’ due to islet disaggregation and islet death. However, these ‘lost’ islets would probably not have survived in the recipient, and a period of islet culture enables more extensive evaluation of the graft pre-transplantation.
The Islet Transplant Procedure
Pre-transplant Preparation
Once a patient has been placed on the waiting list for islet transplantation, they await a suitable donor pancreas for islet isolation and subsequent transplantation. Once the islet yield of the matched pancreas has been confirmed to be sufficient for transplanting into that recipient (usually ≥5000 islet equivalents (IEQ) per kg, where an IEQ is an islet count that has been adjusted to standardize the islet diameter to 150 μm), the patient is admitted to the transplant centre to begin immunosuppressive induction therapy. Prior to the introduction of the Edmonton Protocol in the late 1990s, corticosteroids were a mainstay component of the immunosuppressive protocol. It is widely known, however, that these medications are themselves diabetogenic and are particularly damaging to newly transplanted islets. The Edmonton Protocol provided a steroid-free approach, employing sirolimus and low-dose tacrolimus maintenance therapy after the potent induction agent daclizumab, an anti-CD25 (IL-2R) monoclonal antibody [16]. Since the removal of daclizumab from the market after patent expiry, other T-cell depletional agents have been used including thymoglobulin (antithymocyte globulin), basiliximab (Il-2R antibody) and, more recently, some of the best results have been used with the anti-CD52 alemtuzumab. At some centers, sirolimus is being replaced from post-transplant immunosuppressive protocols by the better tolerated mycophenolate mofetil.
Many centers are employing adjuvant regimens to enhance outcomes. The blockade of tumor necrosis factor α (TNFα) as a means to prevent its inflammatory attack on islets post-transplantation has been used in the form of Etanercept [43, 44] and Infliximab [45]. Exenatide (a GLP-1 agonist) has found use in patients with graft dysfunction, promoting insulin secretion and improving islet function [46, 47]. In fact, the combination of Etanercept and Exenatide may enhance islet engraftment when given in combination [48]. All patients are given broad-spectrum prophylactic antibiotics shortly before the procedure.
The Transplant Procedure
Today, most clinical islet transplants are performed using percutaneous intrahepatic islet infusion via the portal vein under radiological control. However, a few centers still prefer the surgical mesenteric cannulation approach for additional safety.
Portal infusion offers a minimally invasive procedure, accomplished without need for surgery or general anaesthesia, with the ability to regulate glycemic levels through portal insulin delivery [49]. Once at the confluence of the portal vein, islets are infused aseptically under gravity while the portal venous pressure is monitored. Heparin (70 units/kg) is included in the islet preparation to minimize the chance of portal vein thrombosis. While catheter tract bleeding is a known complication, multiple approaches can be used to prevent this including D-STAT [50], coils and gelfoam or microfibrillary collagen (Avitene®) paste. However, although rare, this percutaneous approach still has some potential procedural risks, including portal thrombosis and bleeding [51].
The surgical approach to portal venous access is less commonly used, though it may be necessary if the percutaneous approach cannot be utilized (e.g., large right-sided liver hemangioma). In this instance, a mesenteric vein is cannulated, utilizing complete surgical control to prevent bleeding. However, this approach has the inherent risks of laparotomy/laparoscopy including bleeding, infection, adhesion formation, and wound breakdown/incisional hernia (especially if on sirolimus).
The liver is the currently the preferred transplant site for a number of reasons. It is easily accessible percutaneously, it has high a vascularity supplying sufficient oxygen and nutrients during the revascularization period, and it contains a sinusoidal structure that enables islets to become trapped and engrafted. Having islets placed within the liver also ensures a physiological release of insulin into the portal vein, although compared with the native pancreas, it suffers from a low oxygen content. Moreover, a significant amount of intraportal islet mass is lost immediately post-transplant due to innate immune pathways involving platelet and complement activation.
To date, numerous alternative islet transplantation sites have been proposed and tested, both experimentally and in some cases clinically. These include the kidney subcapsule, the spleen, pancreas, omentum, gastrointestinal wall, immune privileged sites such as the eye and testis/ovary, and the subcutaneous spaces. Though some alternative sites offer advantageous results in experimental models, their feasibility and translation into clinical settings have been limited. Undoubtedly, ongoing experimental and clinical investigation is required to elucidate an optimal islet transplant site with efforts aimed to improve islet engraftment, long-term insulin independence, and transplant outcomes from single donors.
Post-transplant Care
To minimize stress on the newly transplanted islets, tight glycemic control is maintained using an insulin/glucose sliding scale. It is known that islets engraft more readily if they are able to do so in a euglycemic environment [52]. However, apoptotic islets will release insulin, making the patient susceptible to hypoglycemia. To prevent portal vein thrombosis and combat IBMIR (instant blood mediated inflammatory reaction), unfractionated heparin is infused in the postoperative period. Ultrasonography is routinely performed at day one and one week post-transplant to rule out intraperitoneal hemorrhage and ensure patency of the portal vein. In addition to immunosuppressive drugs, patients are discharged home 1–2 days later on a range of post-transplant medications.
Complications
Procedure-Related Complications
Portal vein thrombosis and major hepatic bleeding account for two of the most serious complications associated with the percutaneous approach to islet transplantation [53]. Portal vein is extremely uncommon now, especially as we now transplant purer islet preparations and use heparin in the preparation and also systemically in the patient. The incidence of bleeding from the catheter tract was not uncommon in the early incidences of clinical islet transplantation [53]. Many of these events have all but been eliminated through methods to reduce the catheter tract. The clinical islet transplantation site in Edmonton currently utilizes Avitene® paste to seal and abate the catheter tract. Although segmental vein thrombosis can occur (5% in the above-mentioned series), main portal vein thrombosis is extremely rare. This risk is largely reduced through the administration of unfractionated heparin (70 units/kg) in the islet preparation, as well as through instituting systemic anticoagulation, post-procedure.
Immunosuppression-Related Complications
Corticosteroids used to form the backbone of immunosuppression regimes in early clinical islet transplantation settings and were found to be quite toxic to islets. The success of the ‘Edmonton Protocol’ is attributed to the immunosuppression scheme that utilized the combination of sirolimus, low-dose tacrolimus and daclizumab in an effort to prevent the deleterious effects of calcineurin inhibitors and steroids [54]. In spite of these refinements, most patients returned to modest amounts of insulin despite the elimination of recurrent hypoglycemia by 5 years post-transplant, clearly indicating room for improvement [55]. Moreover, β-cell survival and function are also compromised due to the proximity of the transplanted islets to high concentrations of these drugs in the hepatoportal circulation [56, 57].
Due to the multiple pathways known to contribute to β-cell attrition and the alloresponse to foreign antigens, it is unlikely that a monotherapy will optimize clinical islet transplantation outcomes and lead to single donor recipients [55]. The implementation of highly potent and selective biological agents for the initiation and maintenance of immunosuppression has made significant progress in reducing the frequency of acute rejection, prolonging graft survival and minimizing the complications of these therapeutic schemes [58, 59]. The University of Minnesota reported improvements to single donor success rates as a result of combining anti-inflammatory biologics to maintenance immunosuppression [43, 60]. In addition, peri-transplant insulin and heparin administration greatly increased the success rate of single donor islet transplants from 10 to 40% [61]. Furthermore, the blockade of tumor necrosis factor alpha with etanercept has also enhanced single donor islet transplant outcomes [43, 61, 62, 63, 64].
The successful establishment of an immunosuppressive regimen that promotes self-tolerance is critical for the long-term success of clinical islet transplantation. A tolerizing regimen that utilizes biologics and techniques that selectively target donor-reactive T cells while expanding populations of regulatory T cells, in an ‘islet friendly’ manner will undoubtedly lead to the definitive cure of T1DM.
Islet transplant recipients often have some degree of renal impairment at the time of transplantation, which can be exacerbated by calcineurin inhibitors. This is true even with the low doses used currently, which can be compounded with the use of sirolimus [65, 66]. Consequently, renal function of islet transplant recipients must be monitored diligently. In addition, islet recipients are prone to the more generalized immunosuppressive complications including leucopenia, mouth ulcers, infections, and malignancy.
Clinical Islet Transplantation Outcomes
Since the inception of the Edmonton Protocol, over 750 islet transplants have been performed in over 30 International transplant centers around the world. Unquestionably, the concept of islet transplantation has evolved in a number of countries from an experimental procedure to one that recognized standard clinical therapy.
To date, 677 allogeneic islet transplants have been reported to the Collaborative Islet Transplant Registry (CITR). Of these, 44% were insulin independent at three years post-transplant in the ‘new era’ of islet transplants (2007–2010), as compared to 27% of clinical islet transplant recipients in 1999–2002 [67, 68] (Fig. 14.2a). Moreover, marked improvements in clinical islet transplantation have been observed from 2007 to 2010, as evidenced by retained C-peptide levels, reduction in HbA1c levels and reduced islet reinfusion rates [68]. Shifts in immunosuppression strategies can account for these success rates, though improvements to islet engraftment and subsequent survival are paramount to achieving durable insulin independence.
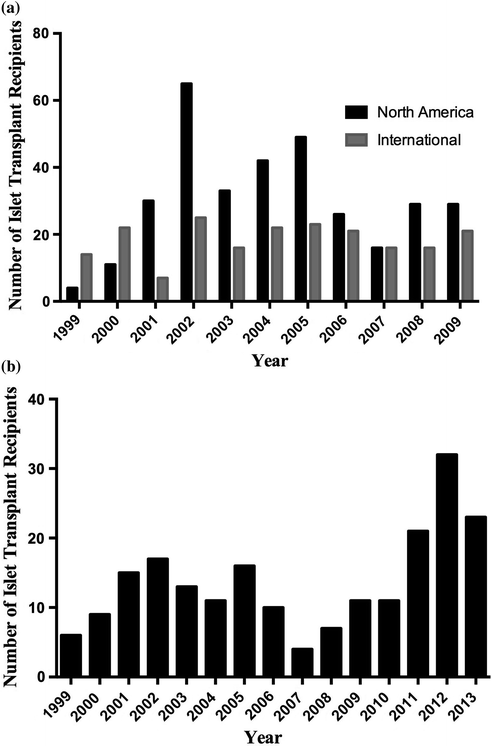

Fig. 14.2
Clinical islet transplant recipients per year. a Number of islet transplant recipients per year completed in North America and Internationally, registered by the CITR. b Number of islet transplant recipients completed by the Edmonton Clinical Islet Transplant Program
In spite of marked improvements in clinical islet transplantation outcomes and substantial transplant activity in international islet transplant centers, few centers are currently active in North America. In the United States, islet transplantation is still classified as an experimental therapy, and as a result immensely lacks the available funds necessary to conduct and support large-scale clinical trials. In an effort to support the FDA biological license application mandate, two pivotal Phase III clinical trials are currently being conducted in specialized islet transplantation centers through the Clinical Islet Transplant (CIT) Consortium (CIT-06 and CIT-07, Clinical Trials.gov NCT00468117 and NCT00434811, respectively). Successful licensure will inevitably recognize islet transplantation as a clinical therapy, thus expanding its therapeutic applicability to patients with T1DM in the United States.
The University of Alberta’s Clinical Islet Transplant Program remains an active site, and in 2013 alone, 66 islet transplants were conducted at the Edmonton site (Fig. 14.2b). The Edmonton Group reports that of over 200 patients transplanted with more than 400 intraportal islet preparations, 79% of recipients continue to show full or partial islet graft function [69]. Notably, the median duration of insulin independence is 34.6 and 11 months for subjects with full or partial graft function. Moreover, the duration of C-peptide is 53.3 and 70.4 months, respectively, for those same patients [70–72].
To date, the application of islet allotransplantation is only suitable for patients with unstable glycemic control that is life-threatening (e.g., hypoglycemia unawareness) and that cannot be corrected by standard conventional and intensive insulin therapies [17]. Patients who exhibit good glycemic control, as well as children, are not currently considered for islet allotransplantation, largely owing to the need for lifelong, chronic immunosuppression. In a recent trial by Ly et al., sensor-augmented pump therapy with automated insulin suspension reduced the rate of moderate and severe hypoglycemia, as well as impaired hypoglycemia awareness over a 6-month period in trial participants. Yet, when compared to the standard insulin pump control group, no change in glycosylated hemoglobin (HbA1C) was observed [72]. Conversely, in islet transplant recipients, HbA1C levels were corrected to levels that could predictably reverse the secondary consequences of diabetes [73]. Moreover, a one-way crossover study conducted by Thompson and colleagues demonstrated that clinical islet transplantation was more effective in reducing progression of diabetic retinopathy and nephropathy than intensive medical therapy [74]. In this therapeutic setting, the lifelong need for immunosuppressive therapy may be readily justified.
Indications and Patient Selection for Islet Transplantation
As noted, the current indications for islet allotransplantation do not include the pediatric population, though the potential applicability to children will be explored below (Table 14.1). Indeed, islet transplantation has been carried out in children, although most have been in the setting of total pancreatectomy and islet autotransplantation for hereditary pancreatitis. In this clinical setting the necessity for immunosuppression is not required.
Table 14.1
Indications and exclusions for islet allotransplantation
Indications for islet transplantation | Exclusions for islet transplantation |
|---|---|
∙ Type 1 diabetes for >5 years ∙ Above 18 years old ∙ Negative stimulated C-peptide (<0.3 ng/ml) ∙ Despite adequate insulin therapy: – Hypoglycemic unawarenessa – Glycemic labilityb – Composite score >75th percentile | ∙ Uncontrolled hypertension ∙ Severe cardiac disease ∙ Macroalbuminuria ∙ Glomerular filtration rate <80 ml/min/1.73 m2 ∙ Potential inability to comply with imunosuppression |
Secondary to the procedure and consequences of the immunosuppressive therapies, there are a number of risks associated with islet transplantation. As such, adult patients selected for islet transplantation must have T1DM with life-threatening complications to justify these risks. Suitable patient populations include those with severe and recurrent hypoglycemic unawareness and/or those with unstable glucose control despite an optimized insulin regime (glycemic lability). The latter includes those requiring hospitalization for hypoglycemia or ketoacidosis. Those with advanced secondary complications of T1DM may also be considered.
In addition to thorough characterization of secondary complications, patient selection also involves the determination of metabolic status. Typically, patients are selected provided there is no endogenous insulin reserve, indicated by the absence of C-peptide. Patients with elevated BMI (>30 kg/m2) or weight > 90 kg may be excluded since the transplanted islet tissue may not meet metabolic demand. The evaluation of hypoglycemia and glycemic lability is assessed through the HYPO score and Lability Index (LI), respectively, developed by Ryan et al. [75]. While the former is based on the frequency, severity and degree of unawareness of the hypoglycemia, the latter is calculated based on the change in glucose levels over time. Patients ranking in the 90th percentile for either score are given consideration for islet transplantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree