Fig. 4.1
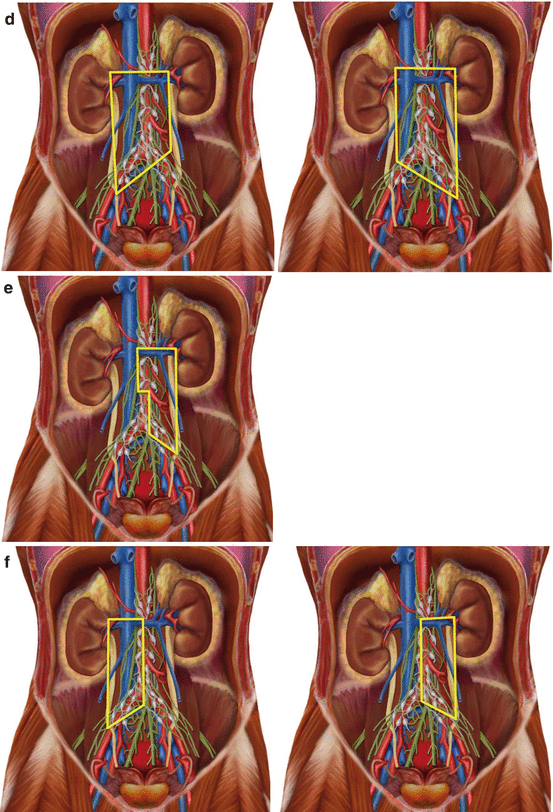
Boundaries of template RPLND according to schools. (a) Original full bilateral RPLND. (b) Modified template dissections (right and left) as proposed at Indiana University. (c) Reduced modified template dissections (right and left) as proposed at Indiana University. (d) Template dissections (right and left) as proposed at Memorial Sloan Kettering Cancer Centre. (e) Modified template dissection (left) as proposed by Weissbach and Bodefeld. (f) Modified template dissections (right and left) as proposed by Pizzocaro
The MSKCC model brought a slight modification to the bilateral dissection template, as only the common iliac contralateral nodes were excluded from the dissection (Fig. 4.1d) [5].
In 1987, Weissbach and Boedefeld proposed, in a large series, a modified RPLND within ipsilateral areas to the side of the tumour as a staging operation for clinical stage I disease. The proposed template was nearly identical to Indiana’s for right-sided tumours; for left-sided tumours, the template was further minimised to eliminate interaortocaval and ipsilateral iliac regions (Fig. 4.1e) [6]. In the same period, Pizzocaro adopted a unilateral template, excluding interaortocaval nodes for left-side tumours, demonstrating that unilateral RPLND was able to offer better functional outcomes without compromising long-term efficacy in patients with stage I NSGCTs (Fig. 4.1f) [7].
4.3 Efficacy of RPLND
4.3.1 Theoretical Advantages of Retroperitoneal Lymph Node Dissection
4.3.1.1 Staging
The traditional purpose of primary RPLND is the accurate staging of retroperitoneal lymph nodes. The historical figures reported that 30 % of patients with clinical stage I non-seminoma and normal markers had metastatic deposits in retroperitoneal lymph nodes [4]. In more recent series, based on CT scans of the modern generation, the rate of patients with metastatic deposits reduced to about 20 % [9], as a reasonable consequence of clinical staging improvement. Actually, none of the available clinical surgical examinations is able to improve the capability of RPLND. Magnetic resonance imaging (MRI) was never proved to replace or improve computed tomography (CT) scans performance. 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) has been evaluated in two distinct multicentric and prospective studies conducted by German centres [10] and by the Medical Research Council [11]. Both studies aimed at increasing the negative predictive value of standard staging with CT scans from about 70–90 % with the use of FDG-PET. The German study included patients at clinical stage I and II-A, who were candidate to primary RPLND. The study was closed when only 72 out of a total of 169 planned patients were accrued before than the accrual was completed, and the achieved negative predictive value was 78 %, with no chance of reaching the expected 90 % rate. The Medical Research Council study provided FDG-PET following CT scans in patients at high risk of recurrence due to the presence of peri-tumoural vascular invasion. One hundred patients with negative PET should have been recruited to rule out a recurrence-free survival (RFS) inferior to 80 % at 2 years. This study too failed its objective and was closed by the internal ethical committee following the accrual of 88 patients, as at 1 year the recurrence-free rate was 65 % only (90 % CI 53–74 %), and the best achievable 2-years RFR could have not be better than 70 %.
4.3.1.2 Eradication of Chemo-Resistant and Chemo-Insensitive Germ Cell Tumours
Small deposits in retroperitoneal nodes can contain teratomatous elements, which cannot be eradicated by adjuvant or therapeutic chemotherapy. Moreover, the necessary time until teratomatous component become clinically evident is potentially very long, implementing the risk of late and advanced relapse. About 21–22 % of patients in the series from Indiana and from MSKCC [12, 13] had teratomatous elements in the resected metastatic lymph nodes following primary RPLND.
4.3.1.3 Simplification of Follow-Up
Following RPLND, relapses usually do not occur in the abdomen and are diagnosed within 24 months since the intervention. In a series from Istituto Nazionale dei Tumori of Milan, less than 2 % of the recurrences were abdominal and only 1.2 % of the relapses occurred beyond the 2nd year of observation [14]. Following surveillance only, the vast majority of relapses occur in the retroperitoneum, a site that conventionally needs CT scan examination, and a not negligible proportion of relapses occur after the 2nd year, especially among patients with no vascular invasion: in a recent multi-institutional series, about 10 % of all recurrences recorded among patients at low risk occurred after the 2nd year [15].
4.3.2 Actual Results According to Pathologic Stage and Recourse to Adjuvant Chemotherapy
When RPLND is used as a staging procedure only, adjuvant chemotherapy could be suggested in case of the presence of nodal metastases at definitive pathology. Actually, an intuitive advantage in recurrence rate is given by adjuvant platinum-based chemotherapy, and the randomised trial published by Williams et al. in 1987 showed a superiority of adjuvant chemotherapy (cisplatin, etoposide and bleomycin (BEP)) versus surveillance alone [18].
One argument in this field was the number of courses to be recommended as adjuvant therapy. Two courses of cisplatin-based chemotherapy became the standard policy, once that this option resulted better tolerated than 3 courses of BEP or 4 courses of EP in case of recurrence, with no difference in terms of recurrences in a randomised German trial [19]. Omission of bleomycin was subsequently demonstrated to be safe, as no difference in preventing recurrences was documented, when 4 × EP was given [20].
On the other hand, controversy still exists concerning the opportunity of adjunctive platinum-based chemotherapy following RPLND in patients with regional nodal involvement as only a proportion of patients with pathological stage II disease experience a recurrence, especially in case of low-volume disease, following a RPLND with curative intent. A risk-adapted strategy could be adopted according to the extent of nodal disease at definitive pathology, unless the patient is not compliant for a surveillance protocol. According to the American Joint Committee on Cancer (AJCC), the probability of relapse for patients with pathologic N1 disease is one third or less, and therefore, observation in compliant patients is considered preferable [21, 22]. For patients with a more advanced stage (pathologic N2), adjuvant chemotherapy is suggested because more than half of these patients will relapse following observation alone [14, 23].
It is a matter of fact that primary RPLND had demonstrated a good capability on cancer control, as demonstrated in a long period experience at Indiana University. Out of a total of 378 with clinical stage I non-seminoma patients, the recurrence rate was 12 and 34 % for pathological stage I and pathological stage II, respectively [24]. Another series by Testicular Cancer Centre Intergroup Study demonstrated a recurrence rate of 10.2 % in pathological stage I patients [25]. These data were confirmed several years later in a large mono-institutional series published by Nicolai et al. that showed a recurrence rate of 12 % in pathological stage I patients and of 32 % in pathological stage II patients following RPLND alone with no adjuvant chemotherapy [26]. The Indiana University experience demonstrated a 35 % recurrence rate in case of pathological stage II disease following RPLND in clinical stage II non-seminoma patients who were followed up only [25]. If we consider only patients with low-volume disease (pathologic N1 according to 1997 TNM classification), the recurrence rate drops out to about 20 % [27].
It has been underscored that as the majority of recurrence occurs within 24 months after surgery, a simplification of follow-up schedule among patients who remain relapse-free following 2 years of surveillance is possible, safe and then advisable.
On the other hand, a European randomised trial published in 2008 showed a significant difference (7.6 %: 95 % CI, 3.1–12.1 %) in terms of recurrence rate in favour of one course of chemotherapy BEP versus RPLND followed by two courses of BEP in case of ascertained nodal metastases [28].
4.4 Morbidity
4.4.1 General Morbidity
Although rare, the possible complications after RPLND include bowel obstruction, lymphocele and chylous ascites, urinary complications (consequent to ureteral damage) and wound infection. The frequency of complications is low in patients undergoing primary nerve-sparing RPLND for clinical stage I non-seminoma in respect of the case of a post-chemotherapy RPLND.
The experience of the German Testicular Cancer Study Group [29] reported on wound infection in 5.4 %, chylous ascites in 2.1 %, bowel obstruction in 2.1 %, urinary complications in 2.5 % and repeat bleeding in 0.8 % of cases. The incidence of complications in this series did not basically differ from the series of the 1980–1990s [30].
In most recent experiences, including data of the 2000s, the complication rate reduced, but the recorded rate of 7 % still remains quite relevant [31, 32]. Laparoscopic RPLND was introduced in 2000s and represented a minimal invasive approach with the intent of providing a reduced morbidity, a shorter hospital stay and a faster recovery of normal function (see further paragraph). Laparoscopic approach reduces the risk of bowel obstruction, wound infection and bleeding; however chylous leak due to lymphatic interruption is one of the most common complications also for this approach, and this is reported in up to 6.6 % of patients following laparoscopic RPLND [33]. In a systematic review of 2008, Rasswailer et al. reported on an overall complication rate of 15.6 % for laparoscopic approach compared to 33 % for open approach [34]. Chylous ascites is a relatively frequent event and may be preventable. A prophylactic low-fat diet has been associated with reduction in incidence of chylous ascites. The Innsbruck group uses a low-fat diet starting 2 weeks before surgery and continuing for 3 weeks following surgery [33].
4.4.2 Preservation of Antegrade Ejaculation
As reported in the previous paragraphs, the preservation of antegrade ejaculation is due to the saving of the anterior postganglionic fibres of the thoracic and lumbar sympathetic chains, which can be obtained with the modification of the template as well as with prospective nerve-sparing techniques.
The retroperitoneal neurologic structures, which transmit the impulse for antegrade ejaculation, include the two paravertebral sympathetic trunks and the postganglionic sympathetic fibres, which travel dorsal to the inferior vena cava and cross ventrally to the aorta. These fibres converge as a trunk, the hypogastric nerve, in the hypogastric plexus on the anterior aorta just caudal to the origin of the inferior mesenteric artery [35]. The elegant studies by Colleselli et al. demonstrated the association between specific post-ganglionic fibres preservation and the chance of maintaining antegrade ejaculation following RPLND, where fibres originating from L3 ganglia represented the most important ones (Fig. 4.2) [36, 37].
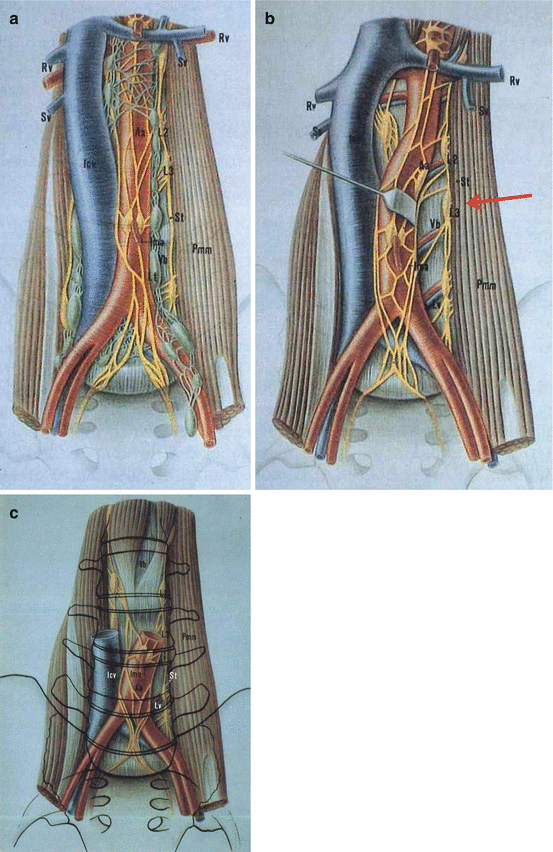

Fig. 4.2
(a) The right sympathetic trunk is located dorsal to inferior vena cava and the left sympathetic trunk is located dorsal and lateral to aorta. (b) L3 ganglion is visible postero-laterally to aorta. (c) Right and left fibers interconnect 2-3 cm caudally to inferior mesenteric artery (IMA) anteriorly to aorta (IVC and aorta are severed 1 cm above IMA) (Reproduced from Colleselli et al. [36])
Nerve-sparing RPLND was firstly described by Jewett in 1987 [38] and soon after by Donohue [39] and represented the culmination of efforts to reduce the morbidity of classic RPLND in patients with testicular cancer. Nerve-sparing RPLND preserves ejaculatory function in “virtually” 100 % of patients with low-stage disease and in selected patients with more advanced disease. As discussed before, crucial help to maintain antegrade ejaculation is given not only by nerve-sparing procedure but also by the development of modified templates. Jewett described the feasibility and effectiveness of the nerve-sparing technique previously described for preservation of antegrade ejaculation. They reported a series of 20 nerve-sparing procedures. The antegrade ejaculation rate was 90 % without a significant impact on the oncological control [40]. Donohue, at Indiana University, demonstrated 100 % preservation of antegrade ejaculation, utilising a similar nerve-sparing approach in a series of 75 patients with a recurrence rate of 28.5 % and 0 % in pathologic stage II and stage I patients respectively [39].
Nonetheless, the preservation of antegrade ejaculation may be eventually guaranteed by the bilateral preservation of postganglionic sympathetic fibres, but a unilateral sparing of these structures is enough to maintain this function. The final common theme in the modified templates was the avoidance of dissection in the region of the contralateral sympathetic trunk and the elimination of contralateral dissection below the inferior mesenteric artery. Since 1981, Indiana University experience began employing modified unilateral RPLND templates for patients with clinical stage I disease (see previous paragraph) [4].
Thanks to the experience of Weissbach [6] and Pizzocaro [7], modified templates were proposed in Europe, providing support to spare the dissection in the interaortocaval and ipsilateral iliac regions for left-sided tumours. In Pizzocaro’s study, the para-aortic region for right-sided tumours and the interaortocaval, precaval and paracaval regions for left-sided tumours were excluded [7].
Richie proposed a full bilateral dissection above the inferior mesenteric artery with only a unilateral dissection inferior to the inferior mesenteric artery. Antegrade ejaculation was maintained in 94 % of the patients undergoing this dissection [41].
Following the introduction of modern concept of nerve-sparing techniques that include both sparing by exclusion and prospective preservation of the retroperitoneal nerves, we documented a 99 % of antegrade ejaculation in patients undergoing unilateral RPLND and in about 95 % of those undergoing bilateral dissection since the 1990s.
4.5 Does RPLND Associate with a High Regional Efficacy? The Issue of In-field Recurrences
As previously discussed, staging RPLND provides good staging results and also a curative potential for low-volume disease. The data that are available in literature are published by referral centres, commonly recognised because of a high number of patients seen per year. The rate of in-field recurrences (i.e. within the boundaries of template dissection) is an important parameter to evaluate the quality of surgery [42].
The study of United States Testicular Cancer Intergroup showed a retroperitoneal recurrence rate of 2.6 % in patients with pathological stage I disease versus 7.5 % of distant recurrences (lung). The Indiana University experience showed only one in-field recurrence in 559 cases (0.19 %) [25].
Low in-field recurrence rate are also reported in European experience as published by Nicolai et al. in 2010. They reported an overall 1.86 % recurrence rate in retroperitoneum and 1.24 % within 2 years from surgery [26].
The relatively high in-field recurrence rate (3.6 %) reported by Albers et al. as the result of a multicentric randomised trial of German Testicular Cancer Study Group [25] confirms that retroperitoneal surgery for NSGCT should be limited to high-volume centres.
4.6 Does RPLND Limit Long-Term Side Effects of Treatment? The Theoretical Long-Term Advantages in Comparison with Other Alternatives
As was seen, RPLND, when performed in the appropriate setting and in experienced hands, is associated with a definite morbidity; a low rate of recurrence, in particular of abdominal recurrences, usually occurring within 2 years of follow-up; and a very high chance of preserving antegrade ejaculation.
Could these advantages be better achieved following an initially nonsurgical approach in clinical stage I non-seminoma? Do we have evident disadvantages following the alternative options of a surveillance programme and of adjuvant chemotherapy?
In the case of active surveillance, a proportion of patients, about 15–20 % in case of low-risk patients and about 30 % in case of a non-risk-adapted strategy, will recur. The vast majority of these relapses, up to near 90 % of cases, will recur in the retroperitoneum [17]. This implies the need of examinations that permit an adequate exploration of the retroperitoneum, which is essentially based on CT scans. In an old series with active surveillance of ours, we recorded that retroperitoneal metastases were larger and occurred significantly later than lung relapses did [43]. This observation is not disavowed in more recent data, as recurrence later than 2 years have been reported in 3 % of series of 223 north-American patients, whose follow-up was just 52 months and having a proportion of 22 % who had not yet reached 2 years of follow-up [44].
Adjuvant chemotherapy as a primary choice associates with a lower risk of recurrence. In the first series, however, the few patients who experienced a relapse (not exceeding 3 %) were difficult to rescue, arising the suspect of a drug resistance induced by chemotherapy. This unfavourable outcome was not fortunately met among the patients recruited by the Swedish and Norwegian Testicular Cancer group, which recently reported on 512 patients undergoing one course of BEP, whose 5-year overall survival is 100 % [16].
One emerging problem with chemotherapy is a growing body of direct and indirect evidence of long-term toxicities. Second cancer, cardiovascular diseases, metabolic syndrome and other effects have been recently addressed.
Second cancer following a first diagnosis of a germ cell tumour has been represented a major concern following a large population study published in 2005 by Travis et al. [45]. Although they reported on an increased 1.8-fold risk of solid tumours after chemotherapy among 10-year survivors, the population included many patients treated as far as in 1943 (i.e. in a period when cisplatin was not administered), the site-specific risks of solid cancers were not reported, and the study essentially focused on long-term effects of radiation therapy. Recently, Fung et al. [46] evaluated the risk of second solid tumour in a target population of 12,691 patients with non-seminoma identified in 16 SEER databases as having received or not having received chemotherapy and who had not undergone radiation therapy between 1980 and 2008. Standard incidence ratio (SIR) was calculated for each subpopulation considering cancer incidence rates in the general population. No increased risk (SIR, 0.93) was recorded following surgery alone, whereas significantly increased 40 % excess (SIR, 1.43) was recorded after chemotherapy. Specifically, median time to second cancer was 12.5 years, and significantly increased 3–7-fold risks of cancers of the kidney (SIR, 3.37), thyroid (SIR, 4.40) and soft tissue tumours (SIR, 7.49) were recorded. Although the absolute excess of a second tumour risk was only 6.47 per 10,000 person-years, this finding arises concerns in delivering chemotherapy in patients who could be managed without chemotherapy, as those presenting as clinical stage I non-seminoma (in the study population, about 30 % of those undergoing chemotherapy had an organ-confined disease).
In general, not specifically addressing the case of stage I disease, treatment with BEP alone had a 5.7-fold higher risk for coronary artery disease compared with surgery only and a 3.1-fold higher risk for myocardial infarction compared with general population, by analysing a series of 990 men treated for testicular cancer between 1980 and 1994 [47]. Increased risks (HR 2.6) for atherosclerotic disease were observed following chemotherapy alone in comparison with surgery alone, while the prevalence of antihypertensive medication was significantly higher following any form of cytotoxic treatment in comparison with surgery alone.
Survivors following a testicular neoplasm treated with cisplatin-based chemotherapy showed an increased risk of developing metabolic syndrome compared with patients treated with surgery alone among 1135 patients aged up to 60 [48]. Although the maximal detrimental effects were observed among patients who received higher dose of cisplatin (>850 mg), this effect was recorded also in those receiving lower doses, and a cumulative dose-event association was documented for cisplatin, etoposide and bleomycin.
Moreover, other long-term side effects have been recently detected among patients undergoing chemotherapy. Fertility is severely impaired by chemotherapy, which, as well as radiation therapy, can significantly compromise the DNA integrity and quality of sperm after treatment [49]. Essentially, infertility following RPLND alone is almost due to the loss of antegrade ejaculation, which currently occurs in a marginal proportion of patients undergoing primary RPLND.
Lauritsen et al. [50] found out that renal function invariably worsens following chemotherapy in patients with metastatic germ cell tumours, and this is usually underestimated with the use of formulae surrogating the direct glomerular filtrate measure with ethylene-diamine-tetra-acetic acid (EDTA).
On the other hand, primary active surveillance still poses questions regarding duration, intensity and modality of follow-up regimen. We do not have a uniform schedule of follow-up, as different respectable plans have been proposed. Three main issues lay under the surveillance strategy. The first is type and number of examinations in the real common practice. In a recent survey performed among Australian medical oncologists, the number of abdominal CT scans, chest CT scans and chest-X rays in a period of 5 years in case of stage I non-seminoma varied from 1 to 15, 0 to 14 and 0 to 27, respectively [51]. The number of abdominal CT scans delivered in the two distinct academic north-American centres experiencing non-risk-adapted active surveillance in stage I non-seminoma deeply differed [44], as one centre doubled (14) the number of CT scans of the other one (7). The second issue is adherence to protocol. Recent data on stage I seminoma reports on a 14 % drop-off at 2 years and of a 38 % at 5 years [52]. We can only hypothesise that in case of non-seminoma, the situation could be better than what we experienced in the past [43]. The third issue regards toxicity of diagnostic radiations. We have evidence that diagnostic radiation doses exceeding 50–100 mSv associate with an increased risk of second cancer [53]. The risk of second cancer increases for each single CT scans delivered and it is greater in younger individuals [54, 55].
Finally, we can address the long-term oncologic efficacy but without any evidence-based answer. As the prognosis of clinical stage I disease is fortunately compressed towards the 100 % of cure rate, it is almost impossible to appreciate any difference in terms of efficacy among the different options. In a large population-based study, RPLND emerged as a favourable risk factor for patients with non-seminoma, giving a 7-fold greater chance of surviving cancer in respect of those not receiving surgery [56]. This datum is importantly hampered by the nature of the study, but it underscores the therapeutic impact of retroperitoneal surgery in this disease.
4.7 RPLND and Prognostic Factors of Recurrence
RPLND has been proposed both as risk-adapted choice and as exclusive treatment independently of risk factors. Following RPLND, we may have an impact on the risk of recurrence, which could still drive further decision.
The simpler risk stratification after RPLND is based on nodal status. Patients with nodal metastases found in the resected specimen may undergo adjuvant treatment as discussed before, usually represented by two courses of PEB. This policy followed the real experience of some decades ago, when the proportion of patients with nodal metastases was greater as also many patients with clinical stage II underwent RPLND and because of a lower clinical staging accuracy.
As a whole, the risk of recurrence in case of nodal disease was about 30 % in the case of pathologic stage II-A/II-B, and it was greater when the burden of retroperitoneal disease was greater. The first dichotomisation in case of nodal metastases has been represented by considering nodal disease extent and proposing adjuvant chemotherapy in case of pathological nodal status pN ≥ 1. The role adjuvant chemotherapy and the evolution of this issue are illustrated in the previous paragraph.
Further clinical researches have been exploring prediction of relapse following RPLND according to more parameters. Evaluation of characteristics of metastatic lymph nodes was intuitive. Number of metastases, presence of extranodal extension, positive nodes ratio and histology of metastases were evaluated by different authors. Beck et al. at Indiana University sequentially evaluated nodal metastases features as predictors of recurrence in 118 patients with nodal stage B1 disease following RPLND. Neither the number of positive lymph nodes (continuous or categorical) nor the ratio of the number of positive lymph nodes to the total number removed (continuous or categorical) predicted recurrence [12]. Similarly, disease-free survival was not statistically different according to each of the solitary histological subtypes (embryonal carcinoma, yolk sac tumour, seminoma and teratoma) or according to embryonal carcinoma presence (pure vs mixed vs absent) [57]. Finally, these authors were unable to detect any prognostic significance of extranodal extension (present vs absent) in a series of 80 patients with nodal metastases [12]. Similar conclusions were reached at MSKCC after the evaluation of 90 patients with nodal metastases at RPLND who did not receive adjuvant treatment. Number of metastatic nodes, embryonal carcinoma predominance, extranodal extension, presence of necrosis and diameter of node did not represent significant predictors of recurrence [58]. A countertrend experience found out that both less than 3 metastatic lymph nodes and a node positive ratio <9 % significantly predict a <10 % risk of recurrence at 2 years, leading to consider that patients with small nodal burden of retroperitoneal disease clinically may behave as patients with negative nodes [59].
Another hypothesis is the integration of pathologic parameters of primary tumour with information regarding the nodal status at RPLND. Vascular invasion, pT category and percentage of embryonal carcinoma component in the primary tumour (as well as nodal metastases at definitive pathology) still remain significant predictors of relapse following RPLND, although the clinical usefulness of such information is limited [8]. Eventually, the proportion of patients still having a clinically relevant risk of recurrence after RPLND is small. In a series of 322 patients undergoing RPLND alone, only 9 % of patients had an individual risk exceeding 30 % of further recurrence (i.e. a risk equal to the pretest probability of recurrence in clinical stage I population) and only 3 % had a risk exceeding 50 % [26]. These proportions limit the usefulness of such predictive information in the clinical practice and underscore the ability of RPLND in modifying the aggressiveness of the disease.
4.8 Randomised Trials and RPLND
Primary RPLND was compared with one single course of PEB in a nationwide prospective randomised trial in Germany [28]. A total of 382 patients were randomly allocated and eventually 173 underwent RPLND and 174 one course of PEB between 1996 and 2005. RPLND template provided unilateral ipsilateral or modified dissection. Patients with nodal metastases at RPLND had to receive two courses of adjuvant BEP chemotherapy, while those with negative nodes were followed. Pathological features at diagnoses were centrally reviewed and about 42 % of patients in each group presented vascular invasion.
Following RPLND, 19.5 % of patients had nodal metastases and received adjuvant treatment. The aim of the study to test performance of both treatments in a community-based acceptance in primary care hospitals was achieved, as actually 61 centres were involved, although 12 centres recruited two thirds of the patients. After a median of 4.7 years, two relapses have been reported following one course of adjuvant BEP and 13 relapses have occurred after RPLND. This was statistically significant in favour of adjuvant PEB with a hazard ratio of 7.94 (95 % CI, 1.81–34.48). None of the patients undergoing adjuvant chemotherapy following RPLND relapsed. Interestingly, 7 of the 13 patients who relapsed after RPLND recurred in the retroperitoneum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree