Transfusional iron loading inevitably results in hepatic iron accumulation, with variable extrahepatic distribution that is typically less pronounced in sickle cell disease than in thalassemia disorders. Iron chelation therapy has the goal of preventing iron-mediated tissue damage through controlling tissue iron levels, without incurring chelator-mediated toxicity. Historically, target levels for tissue iron control have been limited by the increased frequency of deferoxamine-mediated toxicity and low levels of iron loading. With newer chelation regimes, these limitations are less evident. The reporting of responses to chelation therapies has typically focused on average changes in serum ferritin in patient populations. This approach has three limitations. First, changes in serum ferritin may not reflect trends in iron balance equally in all patients or for all chelation regimens. Second, this provides no information about the proportion of patients likely respond. Third, this gives insufficient information about iron trends in tissues such as the heart. Monitoring of iron overload has advanced with the increasing use of MRI techniques to estimate iron balance (changes in liver iron concentration) and extrahepatic iron distribution (myocardial T2*). The term nonresponder has been increasingly used to describe individuals who fail to show a downward trend in one or more of these variables. Lack of a response of an individual may result from inadequate dosing, high transfusion requirement, poor treatment adherence, or unfavorable pharmacology of the chelation regime. This article scrutinizes evidence for response rates to deferoxamine, deferiprone (and combinations), and deferasirox.
Factors contributing to iron overload and its distribution
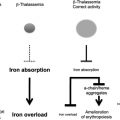
With regular blood transfusion, iron stores increase to many times the norm unless chelation treatment is given. Approximately 200 mg of iron is present in a unit (420 mL) of donated blood, or approximately 1.08 mg of iron per 1 mL of pure red blood cells (ie, hematocrit 1.0). Mean transfusional loading in thalassemia major (TM) is 0.4 mg/kg/d, but this varies. In 20% of patients, this is less than 0.35 mg/kg/d, approximately 60% receive 0.3 to 0.55 mg/kg/d, and a further 20% receive greater than 0.5 mg/kg/d. This transfusional loading is less in sickle cell disease (SCD; 0.22 mg/kg/d) than in TM and is further decreased by approximately 60% when using manual exchanges, whereas neutral iron balance can be achieved with automated exchanges. In myelodysplastic syndromes (MDS), the average rate of iron loading (0.28 mg/kg/d) is less on average than TM. Iron loading may worsen from increased iron absorption caused by increased rates of ineffective erythropoiesis. Thus, iron absorption in thalassemia intermedia (TI) can be up to 5 to 10 times normal, or 0.1 mg/kg/d. Splenectomy seems to increase the rate of gastrointestinal hyperabsorption in TI and other conditions, such as pyruvate kinase deficiency, but interestingly patients with hyposplenic SCD do not hyperabsorb iron.
Tissue iron uptake, in the absence of iron overload, is determined by the distribution of transferrin receptors and by transferrin saturation. However, once transferrin becomes saturated, and with the appearance of plasma iron species that are not bound to transferrin (so-called plasma nontransferrin bound iron [NTBI]), the pattern of tissue iron uptake differs considerably and uptake is mediated through different pathways, such as calcium and zinc channels.
The pattern of tissue iron distribution resulting from transfusional iron overload is best described in TM, in which without chelation therapy, death from iron-induced cardiac failure was usual from the second decade. Postmortem examination in the prechelation era showed high concentrations in liver, heart, and endocrine glands, little in striated muscle, and none in the brain and nervous tissue. Cardiac iron overloading occurred after approximately 70 to 100 units of blood (containing 14–20 g iron) across a range of diagnoses, including MDS. Although cirrhosis has been found in approximately 50% of patients with TM at postmortem, particularly when chronic hepatitis is present, this has historically been an uncommon cause of death, because cardiac disease typically develops first. However, as patients live longer with improved chelation, cirrhosis and hepatocellular carcinoma are likely to increase. Hypogonadism historically occurred in more than half of patients older than 12 years old, leading to disturbances of growth and sexual maturation.
In patients with multitransfused SCD, liver disease is common with cirrhosis in nearly half of the patients who died with severe liver siderosis. By contrast, extrahepatic iron distribution may be delayed in transfused SCD, with MRI showing a lower incidence of myocardial iron deposition, although cardiac iron may be visible postmortem. Lower rates of endocrine complications at matched levels of iron loading to those of patients with TM have also been noted. Possible mechanisms for the lower extrahepatic iron distribution in SCD include lower transfusion rates, less ineffective erythropoiesis, higher plasma hepcidin values, chronic inflammation, and lower NTBI values at matched levels of body iron to those of TM.
Goals of chelation therapy
The primary objective is to maintain body iron at safe levels at all times. Iron stored as ferritin or hemosiderin is not chelated directly at clinically useful rates, so that once accumulated, iron removal is slow and inefficient, relying on the tiny fraction of labile iron that is available for chelation at any moment, either from the breakdown of red cells in macrophages or from the turnover of tissue iron stores in lysosomes. Ideally, chelation therapy therefore should begin before clinically significant iron loading develops. Ample evidence shows that the age at which chelation is started in TM is a key factor in survival, although this is often not accounted for in the retrospective analysis of survival data.
In practice, chelation with deferoxamine (DFO) has traditionally been started only after 2 to 3 years of transfusion or when ferritin exceeds 1000 g/L, for fear of the unwanted effects of overchelation at low levels of body iron (see later discussion). Whether chelation can be safely started earlier with other iron chelators remains to be seen, but this would be desirable. What constitutes safe levels of body iron burden is debated. It may differ depending on the underlying diagnosis and the chelation therapy being used. Preventing the primary accumulation of iron overload in hepatocytes should avoid secondary distribution of iron to endocrine organs and the heart.
The small fraction of body iron that is available in a chelatable form results in only a small proportion of the chelator binding iron before it is excreted or metabolized. Once iron overload has developed, it may take years to reduce body storage iron to safe levels even with the most intensive treatment. Furthermore, iron is removed much more slowly from the heart than from the liver. Consequently, body iron levels that are safe for preventing iron distribution to tissues outside the liver may differ from those that are safe once distribution to these tissues has occurred. Increasing the doses of chelators in an attempt to accelerate iron removal presents a risk for increasing toxicity by chelating iron that is needed for normal tissue metabolism. Therefore, although the slow process of decreasing tissue iron to safe levels is being achieved, a second goal is to make the iron as safe as possible by binding the toxic iron pools responsible for causing tissue damage. Plasma NTBI and labile iron rebound rapidly after a chelator ceases to be present in plasma, so that in principle the continuous presence of chelator is desirable. Continuous chelation therapy also has the potential to minimize the uptake of NTBI species (including labile plasma iron [LPI]) into organs such as heart and endocrine tissues. Whatever the regime, poor adherence decreases exposure to chelation and hence the detoxification of iron. Many studies have shown that adherence has a major influence on outcome with DFO treatment, and this is clearly also important regarding response to oral chelation.
An issue that recently has come into sharper focus is how low the target body iron should be to achieve the optimal balance between iron-induced toxicity and the risk of toxicity from the chelation regime itself. Although the tolerability of DFO clearly decreases as body iron levels fall, this is far from clear with other chelation regimes. Whether toxicity from deferiprone (DFP), with or without DFO, or from deferasirox (DFX) increases at low levels of iron loading is unclear. Evidence suggests that lower levels of iron load can be achieved with these regimes without increasing the risk of these toxicities, and this is likely to impact increasingly on perceived therapeutic goals and guidelines in future.
Goals of chelation therapy
The primary objective is to maintain body iron at safe levels at all times. Iron stored as ferritin or hemosiderin is not chelated directly at clinically useful rates, so that once accumulated, iron removal is slow and inefficient, relying on the tiny fraction of labile iron that is available for chelation at any moment, either from the breakdown of red cells in macrophages or from the turnover of tissue iron stores in lysosomes. Ideally, chelation therapy therefore should begin before clinically significant iron loading develops. Ample evidence shows that the age at which chelation is started in TM is a key factor in survival, although this is often not accounted for in the retrospective analysis of survival data.
In practice, chelation with deferoxamine (DFO) has traditionally been started only after 2 to 3 years of transfusion or when ferritin exceeds 1000 g/L, for fear of the unwanted effects of overchelation at low levels of body iron (see later discussion). Whether chelation can be safely started earlier with other iron chelators remains to be seen, but this would be desirable. What constitutes safe levels of body iron burden is debated. It may differ depending on the underlying diagnosis and the chelation therapy being used. Preventing the primary accumulation of iron overload in hepatocytes should avoid secondary distribution of iron to endocrine organs and the heart.
The small fraction of body iron that is available in a chelatable form results in only a small proportion of the chelator binding iron before it is excreted or metabolized. Once iron overload has developed, it may take years to reduce body storage iron to safe levels even with the most intensive treatment. Furthermore, iron is removed much more slowly from the heart than from the liver. Consequently, body iron levels that are safe for preventing iron distribution to tissues outside the liver may differ from those that are safe once distribution to these tissues has occurred. Increasing the doses of chelators in an attempt to accelerate iron removal presents a risk for increasing toxicity by chelating iron that is needed for normal tissue metabolism. Therefore, although the slow process of decreasing tissue iron to safe levels is being achieved, a second goal is to make the iron as safe as possible by binding the toxic iron pools responsible for causing tissue damage. Plasma NTBI and labile iron rebound rapidly after a chelator ceases to be present in plasma, so that in principle the continuous presence of chelator is desirable. Continuous chelation therapy also has the potential to minimize the uptake of NTBI species (including labile plasma iron [LPI]) into organs such as heart and endocrine tissues. Whatever the regime, poor adherence decreases exposure to chelation and hence the detoxification of iron. Many studies have shown that adherence has a major influence on outcome with DFO treatment, and this is clearly also important regarding response to oral chelation.
An issue that recently has come into sharper focus is how low the target body iron should be to achieve the optimal balance between iron-induced toxicity and the risk of toxicity from the chelation regime itself. Although the tolerability of DFO clearly decreases as body iron levels fall, this is far from clear with other chelation regimes. Whether toxicity from deferiprone (DFP), with or without DFO, or from deferasirox (DFX) increases at low levels of iron loading is unclear. Evidence suggests that lower levels of iron load can be achieved with these regimes without increasing the risk of these toxicities, and this is likely to impact increasingly on perceived therapeutic goals and guidelines in future.
Measures of iron overload and response to chelation therapy
Liver Iron Concentration
Body iron stores are predicted from the liver iron concentration (LIC) using the formula: total body iron stores in mg/kg = 10.6 × the LIC (in mg/g dry weight). Normal LIC values are up to 1.8 mg/g dry weight, and levels up to 7 mg/g dry weight are seen in some nonthalassemic populations without apparent adverse effects. In unchelated patients, high LIC values predict an increased risk of myocardial iron deposition, but once chelation therapy has been initiated, this simple relationship no longer exists. Despite this, high LIC values (>15–20 mg/g dry weight) have been linked to worsening prognosis, liver fibrosis progression, and liver function abnormalities. Measurement of LIC has historically required a liver biopsy of adequate size (>1 mg/g dry weight, >4 mg wet weight, or approximately a 2.5-cm core length). MRI techniques are now available, relying on the general principle that tissue iron exerts a paramagnetic effect on surrounding tissues, affecting the relaxation time of molecules excited by the application of a magnetic field. One method (R2, Ferriscan) is available in a standardized and validated format that is predictive over a clinically useful range, is registered in the European Union and United States, and can use widely available MRI equipment with little extra training of local staff.
Serum Ferritin
Serum ferritin broadly correlates with body iron stores, but in TM, variation in body iron stores accounts for only 57% of the variability in plasma ferritin. This variability is partly because inflammation increases serum ferritin independently of the body iron levels and partly because the distribution of liver iron between macrophages (Kupffer cells) and hepatocytes in the liver has a major impact on plasma ferritin. A sudden increase in serum ferritin should prompt a search for hepatitis, other infections, or inflammatory conditions. Unlike tissue ferritin, serum ferritin is predominantly iron-free and is secreted by macrophages proportionally to their iron content up to values of approximately 3000 μg/L. Greater than this value, iron-rich ferritin tends to leak from hepatocytes, so that responses to treatment may occur at a different rate than at values lower than 3000 μg/L. The relationship between serum ferritin and iron stores is similar in TM and SCD, provided serum values are taken several weeks away from a vaso-occlusive sickle crisis, but in TI, serum ferritin tends to underestimate the degree of iron overloading. The relationship between serum ferritin and body iron stores may also vary with the chelator being used and with the duration of chelation therapy. Despite these caveats, control of serum ferritin lower than 2500 μg/L (with DFO) on a long-term basis is associated with a significantly lower risk of cardiac disease and death. Maintenance of an even lower serum ferritin of 1000 μg/L may be associated with additional advantages.
Monitoring of the Heart
With the development of MRI techniques for estimating myocardial iron, the factors influencing myocardial iron deposition and removal are being increasingly studied. However, the extent to which the rate of iron loading, age of starting iron loading, the underlying cause of iron loading, and the type of chelation therapy on myocardial iron loading are still incompletely understood. In TM, the risk of developing clinically relevant left ventricular (LV) dysfunction increases as the T2 falls below the lower limit found in healthy adults of approximately 20 ms. However, tissue iron correlates inversely with T2 but linearly with 1/T2 (R2), and this latter function clearly shows a linear continuum of increasing left ventricular risk as heart iron rises, rather than a stepwise increase of risk T2 of 20 ms ( Fig. 1 ). Prospective studies have shown that the risk of developing heart failure in the next 12 months rises particularly in patients with T2 values less than 10 ms.
Plasma NTBI and LPI
NTBI is a heterogeneous collection of plasma species unbound to transferrin, some of which are bound to citrate and plasma proteins. Various assays for NTBI provide variable reference ranges but generally correlate with each other. An assay measuring a redox-labile subfraction (the component capable of accelerating oxidation of a flurophore, termed LPI assay ) is suitable for measurements in the presence of chelators. NTBI broadly correlates with transferrin saturation, as does LPI. Weak correlations with serum ferritin and LIC have also been noted, and possible correlations with cardiac iron loading. More recently, clear correlations of LPI with the transfusional iron loading rate have been observed (Porter and colleagues in preparation). LPI values also decline immediately after a single dose of chelator, and progressively with chelation treatments.
Response Rate
Nonresponder is a term that has recently begun to be used in the field of chelation. The term must be used with caution because it implies a fundamental difference between a responder and a nonresponder, which may not necessarily be the case. In this article, the term responder refers to any patient who shows an improving trend in the specified variable of interest (ferritin, LIC, myocardial T2 [mT2]). The term response could also be applied to the attainment of certain desirable thresholds for a given variable (eg, ferritin <2500 μg/L). However, the term responder in this article refers to patients showing improvement rather than reaching a given threshold. In principle, the reasons for nonresponse may relate to the dose prescribed, adherence of patients to treatment, the blood transfusion rate in an individual, or the underlying pharmacology of the chelation regime itself. The latter includes factors such as variability in absorption and metabolism. Changes or lack of changes in ferritin values, although broadly reflecting trends in body iron, can be misleading. A more robust way to measure response is to assess changes in total body iron, calculated based on changes in LIC, over a measured period. However, if only mean population changes are given, it does not inform the clinician about the proportion of patients likely to respond. Fortunately, recent randomized studies have provided some data on iron balance response rates (see later discussion), but these data are only available in prospective studies for mT2 response rates with DFX (70%), because larger studies with other chelation regimes have only reported mean values with DFP monotherapy or DFP with DFO, with individual responses being confined to small numbers of patients.
Chelation regimes to control iron overload
DFO
Chemistry and pharmacology
DFO is a hexadentate chelator binding iron at a 1:1 molar ratio, thus preventing its participation in toxic reactions. In addition to its relatively high molecular weight, the drug is highly hydrophilic, which retards its entry into most cell types except hepatocytes, which seems to have a facilitated uptake mechanism. The iron complex of DFO is highly stable, with good iron scavenging properties at low concentrations of iron or chelator. When DFO is infused subcutaneously at 40 to 50 mg/kg/d, steady-state plasma concentrations are typically no more that 10 μM. Because of the short half-life, levels of the iron-free drug fall to negligible values within a few minutes of stopping infusion, although this takes longer after subcutaneous infusion. Metabolism of the iron-free drug, but not the iron complex, occurs within hepatocytes, so that an increase in metabolites indicates a decrease in the availability of chelatable iron. With 24-hour infusion regimes, the duration of protection from NTBI and labile plasma iron is continuous, but plasma levels still rarely exceed 10 μM at conventional doses, and NTBI is incompletely removed. In contrast, the LPI subfraction of NTBI is effectively removed when chelator is present in plasma.
Iron balance and LIC
Early formal iron balance studies suggested that daily 12-hour infusions at 30 mg/kg could achieve iron balance in TM, particularly if oral ascorbic acid was supplemented at the equivalent of 2 to 3 mg/kg/d. In practice most patients are prescribed DFO approximately five times a week, and under these conditions higher doses are likely to be necessary, but this has only recently been examined systematically (response rates are discussed later). Prospective randomized studies have shown that the percentage of patients experiencing negative iron balance depends on the transfusional loading rate and the dose given ( Tables 1 and 2 ). At typical rates of transfusional ion loading (0.3–0.5 mg/kg/d), negative balance was achieved in 75% of patients prescribed 35 to 49 mg/kg/d given subcutaneously 5 days per week, whereas at doses of 50 mg/kg/d or greater, response rates increased to 86%. At higher transfusional loading rates (≥0.5 mg/kg/d), response was seen in only half of the patients prescribed 35 to 49 mg/kg/d given subcutaneously 5 days per week, but this increased to 89% at doses on 50 mg/kg or greater.
| Dose mg/kg | Low Transfusion <0.3 mg/kg/d | Medium Transfusion 0.3–0.5 mg/kg/d | High Transfusion >0.5 mg/kg/d |
|---|---|---|---|
| 35 to 49 | 76 | 75 | 52 |
| ≥50 | 100 | 86 | 89 |
| Dose mg/kg | Low Transfusion <0.3 mg/kg/d | Medium Transfusion 0.3–0.5 mg/kg/d | High Transfusion >0.5 mg/kg/d |
|---|---|---|---|
| 10 | 29 | 14 | 0 |
| 20 | 76 | 55 | 47 |
| 30 | 96 | 83 | 82 |
Effect on serum ferritin
Dose-dependent reductions with DFO have been recognized for several decades, and the impact of ferritin control on survival are discussed earlier. In general, the trend in serum ferritin often reveals more about compliance and trends in iron balance than do body iron levels at any given time. The impact of dose on ferritin decrements has been shown in large-scale trials. These studies have shown only mean changes, and therefore the percentage of patients experiencing response is unclear.
Effects on the heart
Progressive decreases in cardiac mortality since DFO infusions were first introduced in the late 1970s, together with the reversal of cardiac failure using continuous intravenous DFO, argue persuasively for the clinical beneficial effects. DFO also removes myocardial iron. At very high levels of myocardial iron (T2 values ≤5 ms), a 58% improvement in mT2 was seen over 1 year of intravenous therapy. Improvement in heart function preceded these changes in T2, suggesting that a beneficial effect of DFO on labile iron pools was independent of the slower improvement in T2. With continued infusions, improvement usually continues but may take up to 5 years to normalize. Subcutaneous DFO at standard doses given 5 days per week also improved myocardial iron significantly in patients with mild to moderate myocardial iron loading (10–20 ms) in the context of randomized clinical trials over 1 year. Although both studies used the same methodology, gave similar treatment regimes, and had similar patient baseline characteristics, the change in T2 with DFO differed considerably, being 1.1% change in one study and 2.2% in the second. This finding illustrates the variability that can result from patient selection in different clinics, even when baseline measures of iron load seem comparable.
Tolerability and unwanted effects
Although toxic effects and their management have been described in detail elsewhere, key principles about DFO toxicity are discussed here. First, most of the toxic effects of DFO are dose-related; effects on growth, skeletal changes, and audiometric and retinopathic effects are more likely at higher doses of the drug. In an adult, these effects are unlikely if the dose does not exceed 50 mg/kg and care is taken to reduce the dose as ferritin levels fall (see later discussion). Retinal effects can present with a loss of visual acuity, field defects, and defects in night or color vision. Doses of 100 mg/kg/d, at which these effects were originally described, are rarely given today. Drug-related ototoxicity is typically symmetric and of a high-frequency sensorineural nature. In patients who develop complications, DFO should be temporarily stopped and reintroduced at lower doses when investigations show improvement.
The second principle is that some adverse effects are more likely when iron stores are low; this is particularly clear for neurotoxic complications, with standard doses associated with coma in patients with rheumatoid arthritis without iron overload, and audiometric and retinopathic effects more likely at lower serum ferritin levels, particularly less than 1000 μg/L. The third principle is that some effects limit the dose that can be prescribed to children: doses greater than 40 mg/kg have been associated with an increased risk of impaired growth and skeletal changes in children. Growth retardation was seen when treatment was started early (<3 y) and at higher doses of treatment. Rickets-like bony lesions and genu valgum, in association with metaphyseal changes, particularly in the vertebrae, giving a disproportionately short trunk, can also occur, often with vertebral demineralization and flatness of vertebral bodies radiographically.
Finally, some unwanted effects, such a local skin reactions, allergic reactions, and Yesinia enterocolitica infections, are less obviously dose-related. Management of these is discussed elsewhere.
DFP
Chemistry and pharmacology
This 3-hydroxypyridin-4-one bidentate chelator binds to iron in a 3:1 ratio with a stability constant approximately six orders of magnitude higher than DFO. However, the pM of 20-log of the uncoordinated metal (M, iron) concentration calculated at pH of 7.4, 10 μM ligand, and 1 μM iron (III) is lower than that of DFO at 27.6, and therefore the iron complex is less stable and can donate iron to other ligands, as seen in combined therapy with DFO. DFP has a short plasma half-life of 1.5 hours, and is therefore usually given three times daily. After a single dose, peak plasma concentrations reach approximately 100 μM, with rapid inactivation by glucuronidation in the liver. The low pM implies that the drug will tend to redistribute iron at low chelator or iron concentrations. Consequently, the drug is well suited to shuttling iron when using combined therapies, and will tend to become less efficient at chelating iron as levels of overload decrease.
Effects on iron balance and LIC
In metabolic balance studies, DFP (75 mg/kg) produced a negative iron balance with daily excretion of 0.53 mg/kg/d and excretion mainly in the urine. Meta-analysis of iron balance based on long-term LIC trends from several studies totaling 143 people show considerable variation among studies, reflecting the heterogeneity of dosing schedules, transfusion rates, baseline LIC values, and follow-up periods (response rates are discussed below). The response rates for negative iron balance (decrements in LIC) have not been directly reported in prospective trials, but information can be gleaned from data within some papers. In general, response rates seem to be lower at higher transfusional loading rates, and fall with time and at lower levels of iron load. In a prospective study, 76% of patients with TM showed an LIC response at a mean of 3 years, but this decreased to 56% after further follow-up. In another prospective study over 2 years using LIC measured using a superconducting quantum interference device (SQUID), 24% overall showed response with negative iron balance over 2 years). When analysis is confined to patients with baseline LIC values greater than 9 mg/g dry weight, this response rate increases to 50%. This article also showed lower response rates at higher transfusional iron-loading rates.
Effects on serum ferritin
The effects on serum ferritin have been examined in several randomized trials using DFO as the comparator (combined total, 235 people). The relative effects of the two drugs differ considerably among the studies, which may reflect the different doses of drugs used and differences in baseline characteristics of patients both within and between studies. Pooled analysis shows a statistically significant decrease in serum ferritin at 6 months, in favor of DFO. Ferritin response rates (percentage of patients showing decrements in serum ferritin) may show discrepancies from LIC effects and lower response rates at baseline ferritin values of less than 2500 μg/L. In one study, 83% of patients were responders with respect to serum ferritin at 70 mg/kg for a minimum of 1 year, but only 30% of patients were responders with respect to LIC. A similar discrepancy between responses in LIC and serum ferritin were found in another study in which, although a correlation was seen regarding changes in LIC and ferritin, the response rate for ferritin was considerably greater (58%) than for LIC (24%). It is also clear that the ferritin response is greater at higher baseline values with low response rates when baseline values are less than 2500 μg/L. Response rates (percentage of patients showing an improving trend) have not been reported at doses greater than 75 mg/kg.
Effects on the heart
Initial studies with DFP monotherapy showed continued cardiac mortality, but more recent reports show a declining frequency that several authors have attributed to this chelator being used with or without DFO. A retrospective analysis of survival that included seven Italian hospitals included 359 patients treated by DFO and 157 receiving DFP for a median of 4.3 years. The investigators noted 52 cardiac events, including 10 cardiac deaths, among patients treated with DFO, but no cardiac events in a smaller number of patients who received DFP. However this absence of mortality in the DFP patients contrasted with a larger 3-year prospective study of 532 patients, among whom 11 cardiac deaths (2%) were seen.
In a single-center Italian study, 54 DFP-treated patients were compared retrospectively with 75 DFO-treated patients for cardiac complications and survival. Worsening of preexisting cardiac disease or new onset of cardiac abnormalities occurred in 4% of the patients treated with DFP compared with 20% of the patients treated with DFO. However this was not a balanced comparison, because the three patients who died on DFO were 6 to 8 years older than the mean age of those in the DFP group, and five patients in the DFO group had New York Heart Association (NYHA) class II to IV cardiac disease at outset compared with only one in the DFP group. These differences illustrate the influences of center effects and patient selection on outcome, and the need for prospective randomized studies if relative long-term merits of chelating regimes are to be inferred.
A prospective randomized study has provided persuasive evidence for the cardioprotective effects of DFP. This study compared DFP at 92 mg/kg with subcutaneous DFO at 53 mg/kg given 5 days a week in thalassemic patients with mild to moderate cardiac siderosis (T2 8–20 ms). After 1 year of treatment, although the proportion of patients experiencing response was not given, the average improvement in T2 and increase in left ventricular ejection fraction were significantly greater in those treated with DFP than with DFO.
Tolerability and unwanted effects
Tolerability and unwanted effects have been described in detail elsewhere, but some key principles are discussed. The International Study Group on Oral Chelators found that nausea and other gastrointestinal symptoms, arthralgia, zinc deficiency, and fluctuating liver function tests, especially in patients positive for anti–hepatitis C virus antibodies, were the most frequent complications. No prospective study has compared tolerability of the drug at two doses. However, although the effect of dosing on agranulocytosis and cytopenias in humans is unclear, in animal studies, bone marrow hypoplasia with leucopaenia is dose-related. Systematic tolerability studies in children have not been reported, but a high rate of thrombocytopenia (45%) has been reported in only one study in children younger than 6 years that was reversible on cessation of treatment. Arthropathy may be more common with high levels of iron overload, suggesting a possible effect of the iron complex. The duration of observation may also influence the frequency of arthropathy, because in one prospective study it increased from 6% at 1 year to 13% at 4 years. Whether unwanted effects increase at low levels of body iron, as occurs with DFO, is unclear; however, experience with combined DFP and DFO suggests that toxicity may not be increased at low body iron burdens, although this has not been studied prospectively.
DFP and DFO Combinations
Pharmacology
Combined therapy could provide an advantage through several ways. First, chelators could be alternated to provide continuous exposure to chelation; for example, DFO given every night and DFP during the day. This regimen, in principle, can provide 24-hour removal of LPI. These chelators can also be given at the same time, giving the possibility of a drug interaction through a so called shuttle mechanism, in which iron is chelated rapidly by DFP at sites relatively unavailable to DFO and then donated to the more stable DFO molecules. Experimental evidence has shown this effect in animal models of iron overload, and this shuttling effect was recently shown for the removal of NTBI in the plasma compartment of patients with TM. In practice, sequential use of these chelators is more commonly adopted, but with numbers of days of DFO varying from 2 to 5 days per week.
Effects on liver iron
In a randomized study of 60 patients, the LIC was less than 7 mg/g dry weight at baseline and was on average maintained both with combination and DFO monotherapy. In a prospective randomized study from Turkey, the effect of DFO monotherapy at 40 to 50 mg/kg subcutaneously five times per week was compared with DFO at 75 mg/kg daily or DFP at 75 mg/kg daily plus twice-weekly DFO. The decrease in liver iron was highest in the DFO monotherapy group and lowest in the DFP monotherapy group, with sequential combination treatment showing an intermediate effect. A randomized study in Italian patients compared DFP at 75 mg/kg/d plus DFO five times per week and DFO monotherapy five times a week, and found that the improvement in liver T2 (as a surrogate measure of LIC) was greater in the combination arm. The proportion of patients experiencing response to treatment with a decrease in LIC can be extracted from some articles showing responses in individual patients. In a small randomized study, the LIC response rate at 12 months was 87% with combined therapy compared with only 32% with DFP monotherapy ( Fig. 2 ).