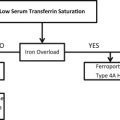
Recent discoveries of mutations in genes leading to inherited diseases of iron overload and iron deficiency have advanced the understanding of iron homeostasis in humans. Articles from Bardou-Jacquet and Brissot, and Heeney and Finberg provide strategies for diagnosis and management of inherited iron overload and inherited iron deficiency, respectively. Inherited sideroblastic anemias are a heterogeneous group of diseases whose phenotype includes the accumulation of mitochondrial iron related to defective heme biosynthesis, iron-sulfur cluster biogenesis, or mitochondrial protein synthesis. In their article, Bottomley and Fleming summarize the heterogeneous phenotypes and the known mutations that explain the disorders in approximately two-thirds of the patients.
Of course, not all iron-related disease is congenital. Acquired iron deficiency is estimated to affect over one billion persons and is the most common cause of the nutritional anemias. Powers and Buchanan review iron deficiency and the dearth of well-designed clinical trials for its treatment and touch on some of the newer parenteral iron therapies. The discovery of hepcidin as the central regulator of iron metabolism has led to intense research into the regulation of hepcidin itself. The effect of chronic inflammation on iron homeostasis and the anemia of chronic inflammation is expertly reviewed by Nemeth and Ganz. The regulatory effect of iron stores on hepcidin is trumped by the ineffective erythropoiesis of the thalassemia syndromes, yet the exact mechanism is unclear. The pathophysiology of acquired transfusional iron overload and the central role of “unchaperoned” iron is reviewed by Porter and Garbowski. Marsella and Borgna-Pignatti then summarize the current use of iron chelation therapy in thalassemia major and sickle cell disease.
The monitoring of iron overload is addressed in the final article. Wood reviews the history of methods of iron assessment and focuses on the use of magnetic resonance as the de facto gold standard for noninvasive multiorgan iron quantification.
Together, these articles provide a detailed view of the remarkable advances in our understanding of the role of iron in human health and disease, and we are very grateful to the authors for their renowned work in this field and their thoughtful contributions to this issue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree