(1)
Institut Bergonié, Bordeaux, France
Abstract
Conduction of nerve impulse is a major signalling pathway in multicellular organisms and requires ion channels situated at the level of plasma membranes of excitable cells. Opening and closing of these channels are controlled by the level of membrane polarisation, and this explains their generic name of voltage-operated channels (VOC). At the level of synapses, nerve impulse transduction from a neuron to another one or to a muscle cell is operated through the release, in the synaptic cleft, of signals that activate the opening of other ion channels. These are called receptor–operated channels (ROC), ionotropic receptors or ligand–gated ion channels (LGIC). Their ligands are neurotransmitters: acetylcholine, serotonin, amino acids and purine nucleotides (ATP). They can in addition react with intracellular proteins (small G-proteins, cytoplasmic tyrosine kinases) and with cytoskeletal proteins. Outside the nervous tissues, they can behave as signalling molecules in many cell types.
Receptor-activated ion channels are also present within the cells and enable especially the transfer of Ca2+ ions from one compartment to another one. Calcium signalling has been mentioned in Chap. 6: the second messenger resulting from GPCR (G–protein-coupled receptors) activation leads to Ca2+ release in the cytosol, which enables this ‘third messenger’ to activate numerous effectors, which play a major role in muscle contraction and secretion processes.
We will not detail here all the aspects of the signalling operated by ionic fluxes, which pertain to neurosciences rather than to oncology; after a simplified presentation of LGIC in excitable cells, we will focus on two special types of channel receptors: purinergic receptors and receptors enabling intracellular Ca2+ mobilisation.
15.1 Activation of Ligand-Gated Ion Channels
Ionotropic receptors or LGICs are ion channels allowing the transfer through the plasma membrane of excitable cells of anions (Cl−) or cations (Na+, K+, Ca2+). They are distinct from voltage-gated ion channels acting upstream or downstream their activation. The main LGICs are the acetylcholine nicotinic receptor, the serotonin receptor, certain receptors of γ-aminobutyric acid (GABA), glutamic acid and glycine, and ATP receptors called purinergic or P2X receptors. For most of these ligands, other receptors exist, which belong to the GPCR family; they are called metabotropic receptors to distinguish them from ionotropic receptors: the acetylcholine muscarinic receptor, amino acid receptors and a second kind of purinergic receptors, called P2Y, which also recognise adenylyl nucleotides, distinct from the adenosine receptors P1 or ADORs.
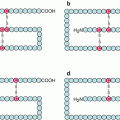
LGICs have a similar general structure, comprising transmembrane proteins that are assembled together to constitute a pore through which ions (anions or cations according to the LGIC) are transferred. There exist about 70 protein subunits constituting precise functional units, localised especially at the level of synapses. Some have a pentameric structure (acetylcholine, serotonin, GABA, glycine receptors), others a tetrameric structure (glutamate receptors) or a trimeric structure (purinergic receptors). The diversity of subunit assembly in each type of receptor (Fig. 15.1) enables the specificity of ligand recognition and binding and determines the ion to be transferred, the conductance of the channel, the speed of opening and closing of the channel, etc.
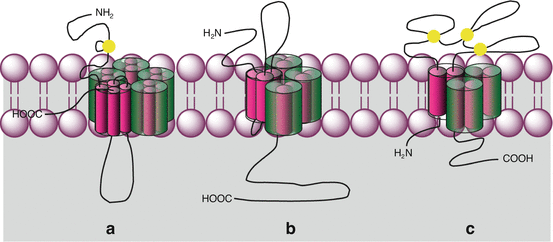

Fig. 15.1
General organisation of ion channel-coupled receptors. Plasma membrane ligand-gated ion channels (LGIC) are of several types. The N-terminal and C-terminal domains can be extracellular or cytoplasmic. Disulphide bridges are indicated in yellow. (a) Pentameric receptors, each monomer being constituted of four transmembrane helices: acetylcholine nicotinic receptor, serotonin, GABA and glycine receptors; (b) tetrameric receptors, each monomer being constituted of three transmembrane helices: glutamate receptor; (c) trimeric receptors, each monomer being constituted of two transmembrane helices: purinergic receptors, P2X type
Acetylcholine and serotonin receptors are Na+, K+ and Ca2+ channels; they induce membrane depolarisation and stimulate the opening of the voltage-dependent ion channels that convey nerve impulses: they are present at the level of excitatory synapses. Glycine and GABA receptors, in contrast, are Cl− channels, which induce membrane hyperpolarisation when open; they have a negative effect on nerve impulse conduction and are therefore present at the level of inhibitory synapses. Finally, the ionotropic glutamate receptors are calcium channels involved in special modalities of synaptic transmission that will not be described here.
The nicotinic receptor of acetylcholine has been one of the first receptors identified, both at the physiological and at the molecular levels; it has clearly left its mark on the history of cell signalling and synaptic transmission at the level of the neuromuscular synapse. It comprises five assembled subunits: two of α type and one of each other type (β, γ and δ). There are nine different genes encoding the α subunits, four the β subunits and one the γ and δ subunits. Each subunit is a transmembrane protein with four membrane-spanning helices (M1 to M4) and extracellular N-terminal and C-terminal domains. The M2 helices interact together to delineate the pore used for cation flux, and the N-terminal domains also interact to delineate the binding site of acetylcholine. The ionotropic receptors of serotonin, glycine and GABA are built according to the same general scheme.
15.2 Purinergic Receptors
There are seven purinergic P2X receptors, P2X1 to P2X7, which are formed of three subunits, identical or different, each made of two transmembrane domains separated by a long extracellular loop containing always ten cysteine residues bound by disulphide bonds, and intracytoplasmic N-terminal and C-terminal domains. Ligand-driven receptor activation induces the entry of Na+ or Ca2+ ions inside the cells and the efflux of K+ ions, as a function of their concentration gradient and without marked selectivity. This results in membrane depolarisation, which is able to activate voltage-dependent channels and to generate an action potential. Purinergic receptors are localised in excitable cells (neurons, nonstriated muscle cells) and also in epithelial cells (lung, intestine, etc.) and endothelial cells.
It had been observed long ago that ATP could display antiproliferative activity. One can hypothesise that this effect is mediated by purinergic receptor (GPCR or LGIC) activation but possibly also by the synthesis of adenosine by membrane ecto-ATPases, since adenosine can bind distinct GPCRs called ADORs, or even by a nutritional effect of ATP. P2X and P2Y receptors are expressed in many cancer types, especially P2X5 and P2X7, which are the most frequently expressed. ATP treatment of cultured cells that express these receptors induces apoptosis, as detected by caspase 3 activation (Chap. 18), although the mechanisms underlying this involvement have not been described. In contrast, metabotropic (P2Y) receptors have a positive effect on cell signalling, which are mediated, as always for GPCR, by the second messengers, cyclic AMP, inositol trisphosphate IP3 and Ca2+ (Chap. 6).
Clinical trials have explored the feasibility of ATP perfusions, but it was not possible to conclude, beyond that step, whether these perfusions could be efficient in oncology. However, therapeutic tools more stable than ATP itself might reveal some interest.
15.3 Ca2+ Signalling
The particular importance of Ca2+ in signal transduction arises from the considerable difference existing between its extracellular concentration (about 1.3 mM) and its cytosolic concentration (around 100 nM). The existence of important Ca2+ stores in cytoplasmic organelles such as endoplasmic reticulum, lysosomes and mitochondria enables the generation of intracellular signals enabling the implementation of rapid and transient reactions. Four successive steps are involved in Ca2+ signalling:
Various stimuli generate signals for Ca2+ mobilisation from extra- or intracellular sources.
These signals activate ion channels that transfer a critical amount of Ca2+ in the cytosol.
Ca2+ activates a series of cytoplasmic effectors which are sensitive to its concentration.
Various active transporters pump out Ca2+ out of the cytosol to restore the initial state.
This general scheme operates in multiple ways within cells as a function of the equipment in adequate proteins at each of these four steps.
15.3.1 Ca2+ Mobilisation Signals
Several types of signals can induce Ca2+ mobilisation from the extracellular space or from the intracellular stores (Fig. 15.2). These signals induce the opening of channels enabling a 5–10-fold increase in Ca2+cytosolic concentration (from 100 nM to 500–1,000 nM).
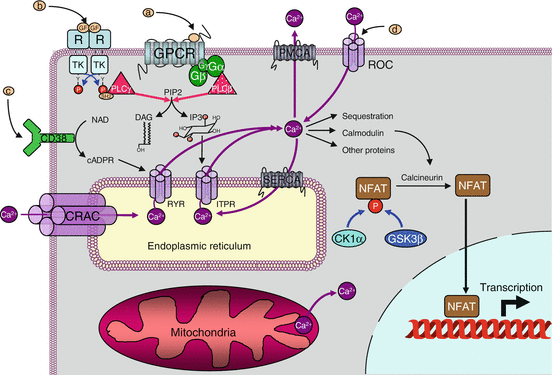

Fig. 15.2
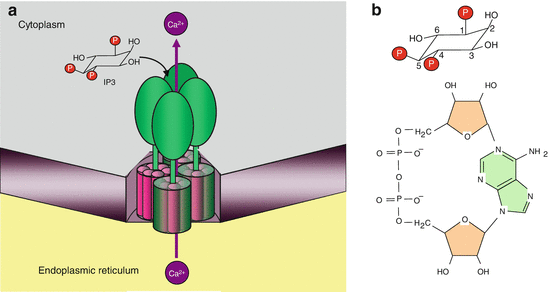
Ca2+ signalling. Cytosolic Ca2+ (100 nM) can come from the extracellular space (1.3 mM) via receptor-activated channels (called ROC or LGIC) or from the endoplasmic reticulum via channels activated by IP3 (ITPR) or cAPDR (RYR). It is pumped back via ATPase transporters of the plasma membrane (PMCA) or the endoplasmic reticulum (SERCA). IP3 is produced especially after activation of a GPCR or a TKR, through the cleavage of phosphatidylinositol 4,5-bisphosphate by PLCβ or PLCγ. cAPDR is produced from NAD by membrane ecto-enzymes, CD38 and CD157. The Ca2+ stores of the endoplasmic reticulum can be reconstituted, when depleted, with extracellular Ca2+, by a direct channel, CRAC, working in connection with an endoplasmic reticulum Ca2+ sensor, STIM. Thus, the signals that can modulate the cytosolic Ca2+ concentration are GPCR ligands (a ), TKR ligands (b), CD38 ligands (c) and LGIC ligands (d), CRAC activation being realised by the depletion of endoplasmic reticulum stores. Among its multiple actions, Ca2+ can activate, via calmodulin, a phosphatase called calcineurin, which activated the NFAT transcription factors
At the intracellular levels, these signals are inositol trisphosphate (IP3, Chap. 6), which acts on specific receptors (IP3R or ITPR) and on cyclic ADP-ribose (cADPR, Fig. 15.3), the physiological activator of ryanodine receptor (RYR). Other signals, such as sphingosine 1-phosphate (S1P) and nicotinic acid adenine dinucleotide phosphate (NAADP), are also active on these poorly characterised receptors. In all cases, Ca2+ itself induces its own release in cytosol thanks to a positive retrocontrol called CICR (Ca 2+–induced Ca 2+ release). The true action of IP3 and cADPR is to increase receptor sensitivity to Ca2+ effects. The same cells can harbour different types of receptors, so that they can respond similarly to different stimuli.