and Winfried G. Rossmanith2
(1)
Lenzkirch, Germany
(2)
Ettlingen, Germany
5.1.3 AKH, RPCH, and HrTH
5.2.3 Enterins
5.3 Kinins
5.3.2 Orcokinins
5.3.3 Leucokinins/Lymnokinins
5.3.5 Sulfakinins
5.4.1 PTTH
5.4.2 PTSH; MIP
5.4.4 GIH, VIH
5.4.5 TMOF
5.4.6 Nebcolloostatin
5.5.2 Corazonin
5.5.4 Eclosion Hormone (EH)
5.5.5 Bursicon
5.6.1 Introduction
5.6.3 Physiology
5.6.4 Phylogeny
5.7.1 Allatotropins
5.7.2 Allatostatins
5.8.1 Introduction
5.8.3 Physiology
5.8.4 Phylogeny
5.9.2 Proctolin
5.10 Summary and Overview
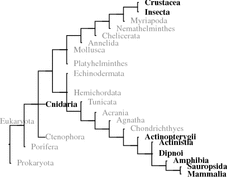
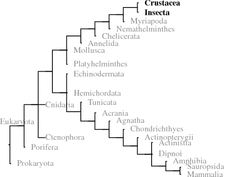
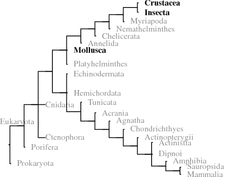
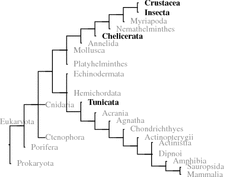
When analyzing invertebrates in the 1930s and 1940s, Berta and Hans Schaller developed the idea of neurosecretion using the large oceanic snail A. californica 1 (Califonia sea hare) with its few and relatively large neurons. Neuropeptides have been found in all metazoans. Where whole genome sequences are available such as in bees A. mellifera 2 ) sequence motifs (signal peptides, cleaving sites of prohormone convertase, terminal glycine) could be investigated. In bees, 200 different potential neuropeptides were identified and the majority of those could be confirmed by chemical analysis (Hummon et al. 2006). In further insects, that is, D. melanogaster or C. elegans,3 the two model organisms of developmental biology, the potential inventory of neuropeptides is identified.
Within deuterostomes some tunicates (e.g., C. intestinalis 4 ) and echinoderms (S. purpurata ) genomes have been fully sequenced. In these species, the focus has been on vertebrate hormone homologues. Since 2008, when we first looked this up, several peptides have been found related to RF-amides, or to GnRH in echinoderms and tachykinins and GnRH in tunicates (Kawada et al. 2011; Roch et al. 2014; Rowe et al. 2014). These hormones have been discussed in chapter 4 Therefore, when we talk about invertebrates in the following, mostly this is about protostomes.5
Table 5.1 summarizes structural motifs and other characteristics of invertebrate hormones. Individual hormones are described in the following sections.
Table 5.1
Sequence motifs of invertebrate neuropeptides
Name | Motif |
|---|---|
Adipokinetic hormones | pELNFx 4∕5-NH2 |
Allatotropin s | targf-NH2 |
Allatostatin s type-A | Y/F-x-FG-L/I-NH2 |
type-B | Wx 6 W-NH2 |
type-C | PISCF-OH |
Bombyxin —insulin-like peptide | B chain → C-peptide → A chain |
CAP | nonapeptides with intramolecular disulfide-bridge like oxytocin/vasopressin |
Enterins | PxxxHxxFV-NH2 |
FMRF /RFamide | FMRF-NH2 or RF-NH2 |
Cardioexcitatory peptide | N D WF-NH2 |
Leucokinin s | FxxWG-NH2 |
Short neuropeptide-F | xxxxRLRF-NH2 |
MIP | PxFy-NH2 (y is F, I, V) |
Orcokinin s | H2N-NxDEI |
PTTH | Cysteine knot like gonadotropins/TSH/NGF |
Pyrokinin s | FxPRL-NH2 |
Sulfakinin s | Tyrosine-sulfated YGHMRF-NH2 |
Tachykinin | FxGLM-NH2 |
5.1 Metabolically Active Peptide Hormones
5.1.1 Crustacean Hyperglycemic Hormone
Fact sheet 5.1: Crustacean hyperglycemic hormone (CHH)
Sequence:
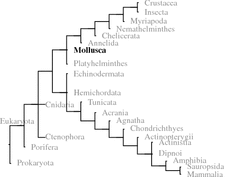
Fig. 5.1.
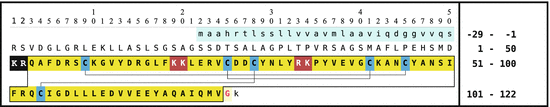

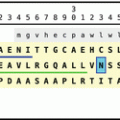
Fig. 5.1
The crustacean hypoglycemic hormone (CHH) of the common European hermit crab (P. bernardus [Pagurus bernhardus]): signal peptide (first line, light blue); the precursor CHH amino acids 1–126 (uppercase) is cut by prohormone convertase (PC) 1 after the motif KR (inverse) to release CHH amino acids 53–126 which is then processed to the amidated CHH (amino acids 53–124 boxed; bold on yellow); unused PC2 motifs are shown white on dark red; black lines between cysteine residues (black on blue) are analogous to Yasuda et al. (1994). The phenylalanine (amino acid 55) may be epimerized; the N-terminal glutamine in the secreted peptide is modified to pyroglutamate (Source: GenBank DQ450960)
Synthesis and target:
CHH and related peptides are formed in neurosecretory cells of the X-organ and-after axonal transport into the neurohemal organ sinus gland-are released into the hemolymph.
Function:
CHH controls carbohydrate metabolism.
Receptor:
Transmembrane guanylate cyclase (mGC).
5.1.1.1 Introduction
CHH of crustacea is a hormone of the X organ in the eye-stalk released in the sinus gland . CHH is the prototype of a peptide family: CHH/MIH /MOIH /GIH /VIH . Crustacean hyperglycemic peptide stimulates the carbohydrate metabolism; molting-inhibiting hormone (MIH) suppresses the molt required for growth in ecdysozoans; mandibular-organ (MO)–inhibiting hormone blocks methylfarnesoate synthesis in the mandibular organ; gonad-inhibiting/vitellogenesis-inhibiting hormones (GIH/VIH) modify gonadal functions.
The CHH homologue of insects, ion transport protein (ITP ), is formed and released in the corpora cardiaca .
5.1.1.2 Biochemistry and Structure
The members of the CHH/MIH/MOIH/GIH/VIH-family (abbreviations in Table 5.2) are peptides with about 80 amino acids with six characteristic cysteines and thus three disulfide bridges (see also Fig. 5.21) . The CHH precursor bears another, CHH precursor-related peptide (CPrP ; amino acids 1–50) whose function remains yet unknown. In crustaceans CHH is formed in the X organ (XO) and released in the sinus gland (SG). The CHH homologue of insects, ion transport protein (ITP), is formed and released in the corpora cardiaca (CC). An alternative ITP was identified by Dircksen et al. (2001) in the pericardial organ. By alternative splicing of the same RNA a C-terminal modified peptide was generated.
Table 5.2
CHH family members
Abbreviation | Name | Alternative name |
|---|---|---|
CHH | Crustacean hyperglycemic hormone | Ion transport peptide (ITP) |
MIH | Molt-inhibiting hormone | |
MOIH | Mandibular organ-inhibiting hormone | |
GIH/VIH | Gonad/vitellogenesis-inhibiting hormone |
5.1.1.3 Physiology
The CHH receptors in crustaceans are transmembrane guanylate cyclases (mCG), which after CHH-binding stimulate intracellular cGMP production. CHH stimulates amylase release in the midgut. This in turn increases sugar content in the hemolymph. CHH (as MIH) may inhibit ecdysteroid biosynthesis which delays molting. ITP similarly binds to an mGC and regulates diuresis in flies.
5.1.1.4 Phylogeny
CHH/ITP and related peptides have been found in chelicerates, nematodes, crustaceans, and insects.
5.1.2 Bombyxin and Insulin-Like Peptides (ILP)
Fact sheet 5.2: Bombyxin and insulin-related peptides (ILP)
Sequence:
Fig. 5.2.
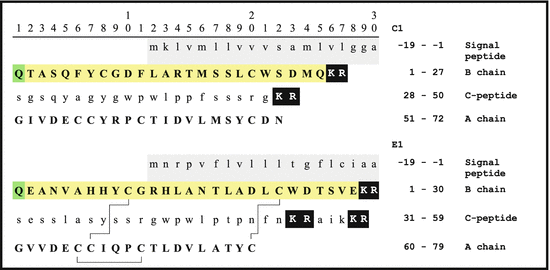

Fig. 5.2
Primary bombyxin sequences from B. mori (Bombyx mori): signal peptide amino acids −19 to −1 in the first row with grey background. Prohormone convertase-1 (PC1 ) cuts off the C-peptide (lowercase) (KR are shown white on black); black lines between cysteine residues of the A- and B-chains indicate disulfide bonds according to Maruyama et al. (1992); N-terminal glutamine (Q) cycled pyroglutamate s (pE, on green) (Source: GenBank P21808, P15410)
Synthesis and target:
Bombyxins/ILPs are synthesized by neurosecretory cells of the CNS, as well as in the gut (in D. melanogaster ).
Function:
Bombyxin/ILPs have important functions for growth.
Receptor:
ILP/bombyxin receptor is (like the insulin receptor) a transmembrane tyrosine kinase. An insulin receptor substrate (IRS) is needed for signal induction.
5.1.2.1 Introduction
Bombyxin s and other ILP s represent neuropeptides involved in regulation of growth, development, fecundity, metabolic homeostasis, and longevity. Nagasawa et al. (1986) identified the small neuropeptide of the prothoracic gland as an insulin of insects.
5.1.2.2 Biochemistry and Structure
The insulin-like peptides show a similar structure to vertebrate insulin. The precursor bears a signal peptide, B-chain, C-peptide, and the A-chain. In contrast to vertebrate insulin, in bombyxin only the PC1 is required for cleaving of the precursor. In addition, the terminal glutamine can be internally closed to the pyroglutamate ring.
B. mori and D. melanogaster each have several ILP genes (Kawakami et al. 1989; Brogiolo et al. 2001), which are expressed in different cells and due to developmental stage differentially regulated. In D. melanogaster seven dILP were identified; these are chronologically and topologically differentially expressed (see Table 5.3).
Embryo | Larva | |
|---|---|---|
dIlp1 | Not expressed (NE) | Not analyzed |
dIlp2 | in midgut, in mesodermal stages 12–16 | In imaginal disksa, in 7 neurosecretory cells in partes intercerebrales (PI) and in salivary glands |
dIlp3 | NE | In 7 neurosecretory PI cells (see dIlp2) |
dIlp4 | in mesodermal stages 2–6, in the anterior midgut rudiment | In midgut |
dIlp5 | NE | In 7 neurosecretory PI cells (s. dIlp2), in gut |
dIlp6 | NE | in gut |
dIlp7 | Ubiquitous but yolk sack, in midgut | In 10 neurons of the ventral nerve cord |
5.1.2.3 Physiology
ILP s/Bombyxin s control indispensable growth functions of insects. Synthesized by a few neurosecretory cells in the pars intercerebralis , ILPs are released in the corpora cardiaca . Release depends on available food.
The insulin receptor of flies (coded for by a singular gene and alternatively spliced to different proteins) is related to the vertebrate IR. In contrast to this IR, an analogous insulin–receptor substrate (IRS) phosphorylated upon ligand binding and thus initiating signal transduction is integrated into the receptor protein chain. However, a separate IRS gene exists in D. melanogaster which codes for chico, which when expressed and phosphorylated initiates further signal cascades. The insulin receptor (InsR) is indispensible in insects. Without the InsR irreparable developmental defects of the fly nervous system occur (Fernandez et al. 1995). To which degree insulin signaling influences growth and differentiation is discussed later. Receptor knockout is lethal for the embryo.
A special function has been reported for the Ilp1 of bees . Expression of Ilp1 is due to feeding of the larva by royal jelly thus fed as a queen to be. Only when royal jelly has been fed, Ilp1 is expressed with the consequence that special checkpoints in development are taken that ultimately lead to the raising of a new queen (Wheeler et al. 2006).
5.1.2.4 Phylogeny
ILPs appear ubiquitously in eumetazoans.
5.1.3 AKH, RPCH, and HrTH
Fact sheet 5.3: Adipokinetic hormone (AKH), red-pigment–concentrating hormone (RPCH), hypertrehalosemic peptide (HrTH)
Sequence:
Fig. 5.3.


Fig. 5.3
Primary sequence of the adipokinetic hormone (AKH) of Periplaneta americana. The signal peptide is shown on gray background, the AKH peptide boxed and on yellow background, and the PC1 motif black on white (Source: GenBank AAV41425)
Synthesis and target:
AKH is formed in the corpora cardiaca by neurosecretory cells. It acts on GPCR on adipocytes of the fat body and stimulates protein kinase A.
Function:
AKH controls metabolism pathways providing energy for flying.
Receptor:
Heptahelical G-protein-coupled transmembrane receptor.
5.1.3.1 Introduction
Adipokinetic hormones (AKH ) are released in the corpora cardiaca of insects and target the fat body. They induce release of sugars and lipids that can be used by equally AKH-stimulated wing muscle cells providing the required forces for flying.
5.1.3.2 Biochemistry and Genes
AKH originates from a preproprotein precursor. At first the signal peptide is cleaved off by the signal peptidase , afterward, by PC1 or PC2, the AKH associated peptide(AAP ) (Fig. 5.3); finally, the C-terminal glycine is oxidized to amide by peptidylglycine alpha-hydroxylating monooxygenase (PHM) and the N-terminal glutamine transformed into Pyroglutamate. Characteristic for AKH are the N-terminal ELFN sequences. Some locusts have been shown to express several AKH peptides (Table 5.4).
Table 5.4
Several adipokinetic hormones in insects
Species | Hormone | Sequence | Dibasic motif | GenBank entry |
|---|---|---|---|---|
L. migratoria | AKH I | pELNFTPNWGT-NH2 | GKR | P55319 |
AKH II | pELNFSAGW-NH2 | GRR | P08379 | |
AKH III | pELNFTPWW-NH2 | GKR | P19872 | |
Schistocerca gregaria | AKH I | pELNFTPNWGT-NH2 | GKR | P18829 |
Schistocerca nitens | AKH II | pELNFSTGW-NH2 | GRR | P53807 |
M. sexta a | AKH | pELTFTSSWG-NH2 | GKR | P67788.1 |
D. melanogaster | AKH | pELTFSPNW-NH2 | GKR | P61855 |
C. maenas b | RPCH | pELNFSPGW-NH2 | GKR | Q26324 |
5.1.3.3 Physiology
After AKH binding to the heptahelical G-protein coupled transmembrane receptor in adipocytes of the fat body the protein kinase A is stimulated by cAMP . This enzyme phosphorylates the lipid storage droplet protein-1 (LSDP-1) and the triglycerol lipase (TG lipase). Both events induce rapid lipid release into the hemolymph (Patel et al. 2005).
Red-pigment–concentrating hormone (RPCH ) of crustaceans is built like AKH. Some X organ neurons secrete RPCH in the sinus gland of the eye stalks. In addition, RPCH/AKH is expressed in the stomatogastric ganglion . Chung and Webster (2004) have shown that RPCH was initially found in X-organs/sinus glands, but was formed in other neurons, presumably in the postcommissural organ. RPCH seems relevant for the rhythmic movements in the gastrointestinal tract (the pyloric rhythm , gastric mill) controlled by 28 neurons of the stomatogastric nerve system (Thirumalai and Marder 2002).
Recently RPCH was also found to be a modulator of the heart ganglion in Cancer borealis (Cruz-Bermudez and Marder 2007). Because different peptides and further substances influence the pulses of heart beating the author suggests that due to expression in the heart ganglion an accelerated distribution of hormones in a given animal appears feasible and thus a shortening of the response time to hormonal release.
5.1.3.4 Phylogeny
Thus far AKH /RPCH have been found in crustaceans and insects.
5.2 Regulation of Heart Frequency and Pressure by Neuropeptides
5.2.1 Cardioacceleratory Peptides: CAP
Fact sheet 5.4: Cardioacceleratory peptide (CAP)
Sequence:
Fig. 5.4.
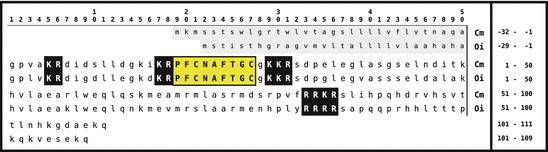

Fig. 5.4
Primary sequences of crustacean cardioacceleratory peptide (CCAP) of Carcinus maenas and Orconectes immunis. The signal peptides are shown on gray background; CAP are boxed and in yellow. Dibasic peptide motifs are shown white on black (Source: GenBank ABB46291 and ABB46293)
Synthesis and target:
CAP are neuronally synthesized in insects and crustacea and released in neurohemal organs.
Function:
CAP stimulates heart frequency.
Receptor:
CAP receptor is a heptahelical GPCR.
5.2.1.1 Introduction
The first cardioacceleratory peptide of crustaceans (CCAP ) has been identified by Stangier et al. (1987) in the pericardial organ . Injections of this peptide increased the heart frequency . CAP has also been found in insects and molluscs. These molecules resemble the posterior pituitary hormones such as oxytocin or vasopressin .
5.2.1.2 Biochemistry and Structure
CCAP are nonapeptides with an intramolecular disulfide bridge . At the C-terminus these peptides are amidated. With the genome of D. melanogaster fully sequenced Park et al. (2002) expressed vasopressin receptor-like GPCR in cell lines and observed calcium mobilization or cAMP enhancement when using CCAP as ligand. However, the CCAP concentrations required for these stimulations were very high which made a specific interaction of CCAP with these receptors unlikely and suggested an unspecific stimulation.
5.2.1.3 Physiology
CCAP in crustaceans and insects has not only been found in the pericardial organ, but in several brain neurons (e.g., in the tobacco hornworm (M. sexta ) in the subesophageal , thoracic , abdominal , and terminal ganglia (Loi et al. 2001). In crustaceans Trube et al. (1994) found CCAP in neurons and neurosecretory cells of the ventral nerve cord . Veelaert et al. (1997) observed neurosecretory cells of the pars intercerebralis of the locust brain secreting CAP into the corpora cardiaca, therein stimulating AKH release, an analogy to the hypothalamic–pituitary axis.
CCAP is preferentially expressed during late stages of molting when the CCAP content in the hemolymph increases (Phlippen et al. 2000; Gammie and Truman 1999; Loi et al. 2001). In order to meet the enhanced water uptake during molting CCAP is thought to provide the required stimulation of heart frequency and thus increased pumping. Gammie and Truman (1997) have additionally demonstrated (again in M. sexta), that CAP induces those essential movements during the molt, resulting in breaking of the old cuticle and in its stripping off. In D. melanogaster Davis et al. (2007) provided evidence of CAP activating those enzymes coloring the newly built skeleton: tyrosine hydroxylase (TH) and DOPA carboxylase , which are dealt with in more detail in the chapter on vertebrate catecholamine biosynthesis.
CAP of molluscs (M-CAP) are related to the CCAP: cyclic peptides with some amino acid exchanges (Table 5.5). Vehovszky et al. (2005) have shown that M-CAP influences the central motor of ingestion.
Table 5.5
Cardioacceleratory peptides (M-CAP ) of molluscs, compared to a CCAP (Source Vehovszky et al. 2005)
Species | Hormone | Sequence |
|---|---|---|
Helix pomatia | M-CAP I | PF 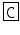 NSYG NSYG 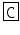 YNS-NH2 YNS-NH2 |
M-CAP II | LF 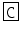 NGYGG NGYGG 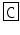 QNL-NH2 QNL-NH2 | |
Orconectes immunis | CCAP | PF  NAFTG NAFTG 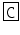 -NH2 -NH2 |
5.2.1.4 Phylogeny
So far CAP have been observed in molluscs, crustaceans, and insects. The presence of a GPCR of the vasopressin family does not allow extrapolating an expression of CAP/vasotocin/vasopressin-like molecules in all bilateria.
5.2.2 Cardioexcitatory Peptide, NDWF-Amide
Fact sheet 5.5: Cardioexcitatory peptide N D WF-NH2)
Sequence
N D WF-NH2; unknown precursor protein.
Synthesis and target
N D WF-NH2 positive brain neurons target cardiovascular muscles in molluscs.
Function
N D WF-NH2 stimulates blood flow and increases blood pressure in molluscs.
Receptor
(unknown).
The molluscan cardioexcitatory peptide is a tripeptide with a D-amino acid : D-tryptophan (DW): asparaginyl-D-trytophanyl-phenylalanyl-amide (N D WF-NH2). Morishita et al. (1997) used it to stimulate in A. kurodai 6 heart contraction , but not heart frequency. Later it was found in A. californica that not only in the heart, but in all tissues with N D WF-positive neurons muscle contractions can be induced by this peptide. The peptide was further identified in snails (Morishita et al. 2003a,b).
D–Amino acids In ribosomes amino acid incorporation is restricted to L-amino acids. The observation of D-isomers presupposes a posttranslational isomerization by a racemase or isomerase . D-Aspartate has been shown to be an neurotransmitter, as well as D-serine. Further D-amino acids incorporated into proteins were found in the animal kingdom. Isomerase acts mainly close to the N-terminus. The spider enzyme (from Agelenopsis aperta ) uses an LXF motif as a substrate and isomerizes the x amino acid; the NXF of molluscs is closely related to the spider motif.
Neither the gene coding for N D WFamide, nor the enzyme isomerizing the tryptophan have thus far been identified (see also the box “D-amino acids”). N D WFamide has thus far been found only in molluscs.
5.2.3 Enterins
Fact sheet 5.6: Enterins
Sequence:
Fig. 5.5.

Fig. 5.5
Primary sequence of the enterins -preproprotein from A. californica . The signal peptide is shown on gray background; the enterins are boxed and with gray background. Monobasic and dibasic peptide motifs are shown white on black. C-terminal glycines are usually oxidized to amides. N-terminal glutaminyl amino acids are internally cycled to pyroglutamate (Origin: Furukawa et al. 2001 GenBank Q95P23)
Synthesis and target:
Enterins are formed in cerebral and buccal ganglia of molluscs.
Function:
Enterins are effective in aorta and gut and inhibit muscle contraction.
Receptor:
(thus far unknown).
5.2.3.1 Introduction
Enterins were identified as characteristic neuropeptides of aplysia (A. californica and A. kurodai ) (Furukawa et al. 2001). No homologue has ever been found in other phyla. Enterins in concert with AMrP are antagonists of N D WF of molluscs and inhibit contractions of the aorta as well as of the gut .
5.2.3.2 Biochemistry and Structure
The enterin -precursor protein is relatively large. Dibasic and monobasic cleavage sites for prohormone convertases are present . The peptides shown in Fig. 5.5 boxed have actually been isolated. The enterin peptide motif is PxxxHxxFV-NH2 . The nonactive additional peptides have been isolated as well, which support the model of precursor cleavage as shown in Fig. 5.5 (Furukawa et al. 2001).
5.2.3.3 Physiology
Enterins inhibit gut contraction s either as neurotransmitters or in an endocrine manner. Their presence in cerebral and buccal ganglia suggests a role in food ingestion. Whether enterins exhibit additional regulatory functions is an open question (Furukawa et al. 2001).
5.2.3.4 Phylogeny
Enterins have only been identified in molluscs.
5.2.4 Mytilus Inhibitory Peptides (MIP; AMrP)
Fact sheet 5.7: Mytilus Inhibitory Peptides
Sequence:
Fig. 5.6.
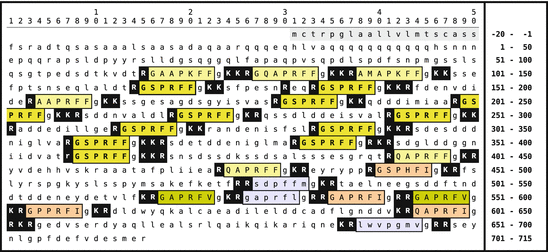

Fig. 5.6
Primary sequence of mytilus inhibitory peptide (MIP, AMrP ) from A. californica . The signal peptide is shown on gray background; the MIP are boxed and with colored background. The prevalent peptide GSPRFF-NH2 is shown in uppercase. Monobasic and dibasic peptide motifs are shown white on black. C-terminal glycines are oxidized to amides. N-terminal glutaminyl amino acids may be internally cycled to pyroglutamate (Origin: Fujisawa et al. 1999; GenBank AAF80382)
Synthesis and target:
MIP are neuropeptides in several ganglia, especially the pleural and abdominal ganglia.
Function:
MIP inhibit muscle contractions.
Receptor:
(thus far unknown).
5.2.4.1 Introduction
MIP were first found in the pedal ganglia of the clam Mytilus edulis (Hirata et al. 1988). In the meanwhile related peptides were observed in other mussels and in snails. The characteristic feature of MIP is inhibition of muscle contractions. .
5.2.4.2 Biochemistry and Structure
The characteristic motif of MIP and related, “aplysia-MIP–like peptides” (AMrP ) is PxFF/I/V-NH2 . The precursor protein of A. californica (Fig. 5.6) encloses 24 peptides, six or seven amino acids long; 21 of these contain this motif, and two others bear a methionine or leucine at the last position. gsprff-NH2 is found elevenfold.
5.2.4.3 Physiology
MIP/AMrP neurons were observed in several ganglia, most prominent in pleural and abdominal ganglia, additionally in cerebral , buccal , and pedal ganglia . MIP/AMrP inhibit muscle contraction s: contractions of the esophagus , of the Penis retraction muscle , or body wall muscle were differentially inhibited by several A. californica -AMrP. Most active were GAPFRV-NH2, GAPFRI-NH2, and GPPFRI-NH2 (Fujisawa et al. 1999). In A. kurodai Sasaki et al. (2004) analyzed stimulation of the aorta vasoconstrictor muscle by N D WFamide and its inhibition by enterins and AMrP (GSPRFF-NH2). In cultivated pleural ganglion neurons McDearmid et al. (2002) identified a 4-aminopyridine-sensitive potassium channel that could be stimulated by GAPRFV-NH2 and GSPRFF-NH2.
5.2.4.4 Phylogeny
To date MIP/AMrP seem to be restricted to molluscs. So-called myoinhibitory peptides (MIP = leucomyosuppressin) from the prothoracic gland of insects are structurally unrelated to the molluscan MIP.
5.2.5 Diuretic Hormones; (DiuH)
Fact sheet 5.8: Diuretic Hormones (DiuH)
Sequence:
Fig. 5.7.


Fig. 5.7
Primary sequence of DiuH from Manduca sexta.The signal peptide is shown on gray background; the diuretic hormone is boxed and on yellow background. Dibasic peptide motifs are shown white on black. The C-terminal glycine will be oxidized to amide (Origin: Kataoka et al. 1989 GenBank P21819)
Synthesis and target
DiuH are formed by neurosecretory cells of the brain and of the corpora cardiaca and act on GPCR in malpighian tubules.
Function:
DiuH stimulate sodium chloride secretion and water transport.
Receptor:
A GPCR of the secretin receptor family.
5.2.5.1 Introduction
Diuresis in insects takes place in the malpighian tubules (MT ), appendices to the mid gut. When a mosquito or a bug has fed on a blood meal, the animal or human blood is hypotonic compared to the insect hemolymph: approximately 280 mOsm in blood and about 370 mOsm in the hemolymph. By secreting sodium and chloride ions into the lumen of the MT and simultaneous uptake of water the food becomes isotonic for the insect and can be ingested and digested. These processes are stimulated by diuretic hormone .
Vasopressin/oxytocin-like peptides as well as CRH-related peptides have been found active as DiuH,8 furthermore there exist some calcitonin-related diuretic peptides . Because they are analyzed in great detail, CRH-like insect peptides and calcitonin-related peptides are regarded as the DiuH being in vitro much more active as inotocin or similar peptides (see Gaede 2004).
5.2.5.2 Biochemistry and Structure
DiuH are formed by the action of the PC1 (and PC2) from precursor peptides. They are C-terminally amidated. Whether the associated peptides (see Fig. 5.7) are physiologically active is not known. In some species some genes were identified as coding for active peptides with amino acid chain lengths of 30 to 50. A differential expression has not been described in the literature. Apart from DiuH, mainly kinins and CAP are stimulators of diuresis.
5.2.5.3 Physiology
DiuH are formed in neurosecretory cells of the pars intercerebralis and of the corpora cardiaca and released thereof. In thoracic and abdominal ganglia an however much reduced expression could be observed (Audsley et al. 1997). DiuH acts via the hemolymph on receptors at the malpighian tubules which in turn increase intracellular cAMP . A similar enhanced intracellular cAMP formation was observed in DiuH receptor expressing cell lines (Gaede 2004).
Because the DiuH content in adult locusts was much reduced on the day after the final molt, as after a feeding, a role of DiuH in the postmolting processes was suggested.
5.2.5.4 Phylogeny
Diuretic hormones have been found in 20 different insect species, but not in other phyla.
5.3 Kinins
Named as kinins we describe neuropeptides and neurohormones isolated due to their myotropic or gut-stimulating properties. Different kinin families can be differentiated by characteristic sequence motifs: pyrokinins/pheromonebiosynthesis–activating neuropeptide (FxPRL-NH2 10), leucokinins/lymnokinins (FxxWG-NH2), sulfakinins (sulfated RF-NH2) , or orcokinins (N-terminal NxDEI peptides .
5.3.1 Pyrokinin/Myotropin and Pheromone Biosynthesis Activating Neuropeptide (PBAN)
Fact sheet 5.9: Pyrokinins/PBAN
Sequence:
Fig. 5.8.


Fig. 5.8
The pheromone-biosynthesis–activating neuropeptide (PBAN) and diapause hormone (DiaH) primary sequence from Bombyx mori. The signal peptide is shown on gray background, the diapause hormone [1–24] and PBAN [102–132] are boxed on yellow/orange background. Monobasic and dibasic peptide motifs are shown black on white. C-terminal glycines are oxidized to amides. Three additional neuropeptide (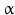 -SG [74–80],
-SG [74–80], 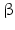 -SG [83–99], and
-SG [83–99], and 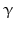 -SG [108–115]) are equally released by prohormone convertases. N-terminal glutamine amino acids are internally cycled to pyroglutamate (Origin: Sato et al. 1993; GenBank P09971)
-SG [108–115]) are equally released by prohormone convertases. N-terminal glutamine amino acids are internally cycled to pyroglutamate (Origin: Sato et al. 1993; GenBank P09971)
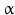 -SG [74–80],
-SG [74–80],  -SG [83–99], and
-SG [83–99], and  -SG [108–115]) are equally released by prohormone convertases. N-terminal glutamine amino acids are internally cycled to pyroglutamate (Origin: Sato et al. 1993; GenBank P09971)
-SG [108–115]) are equally released by prohormone convertases. N-terminal glutamine amino acids are internally cycled to pyroglutamate (Origin: Sato et al. 1993; GenBank P09971)Synthesis and target:
PBAN is formed in the subesophageal gland and released in the corpora cardiaca.
Function:
PBAN stimulates pheromone biosynthesis.
Receptor:
(yet unknown).
5.3.1.1 Introduction
PBAN is formed by singular neurosecretory cells of the subesophageal gland and released in this gland or, after axonal transport, in the corpora cardiaca . PBAN acts on the enzymes of the pheromone glands. The presence of PBAN has been proven in lepidopterans, however, in other insects there are PBAN-like peptides; the analogous peptide of D. melanogaster is called “hugin.”
5.3.1.2 Biochemistry and Structure
Pyrokinin s constitute a group of peptides characterized by a C-terminal FxPRL-NH2 motif and exerting muscle contracting (myotropic) activity. Particular pyrokinins are the PBAN where precursor sequences have only been identified in lepidopterans (Figs. 5.8, 5.9).


Fig. 5.9
The pyrokinin precursor of the honeybee (Apis mellifera). The signal peptide is shown on gray background; pyrokinin s are boxed, in uppercase and on yellow background. Whether the peptides [111–130] and [134–142](in lowercase) are active as pyrokinins is not reported in the literature; Audsley and Weaver (2006) do not mention them. Monobasic and dibasic peptide motifs are shown white on black. Glycines at the C-terminus are always oxidized to amide. N-terminal glutamines may be internally cycled to Pyroglutamate (Origin: GenBank NP_001104182)
The precursor of the silk moth (B. mori ) contains in addition to PBAN the DiaH11 , a pyrokinin, too. Whereas in the silk moth diapause hormone and PBAN are coded for by the same gene, D. melanogaster pyrokinins are found within the hugin , the capa and the cardioacceleratory peptide Cap2b genes (Kean et al. 2002; Baggerman et al. 2002; Meng et al. 2002). In the mosquitoes A. aegyptii and A. gambiae 12 there are three short FxPRL-amide and a single PRL-amide peptide found in a pyrokinin precursor (GenBank Q16N80 and Q7PTL2). The PBAN neuropeptide proprotein of the moth Agrotis ipsilon includes a diapause hormone, where the leucine-amide is replaced by isoleucine-amide, PBAN, and two additional pyrokinins; in the oriental tobacco budworm (Helicoverpa assulta ) DiaH and PBAN and two further pyrokinins are present in the precursor.
5.3.1.3 Physiology
Originally pyrokinins were found due to their myotropic activity. They further influence contractions of the locust oviduct , diapause of the eggs of the silk moth (diapause hormone; Fig. 5.8), acceleration of pupation in larvae of flesh flies, or melanization and reddening in larvae of moths (Torfs et al. 2001). Ectopic (over-)expression of the hugin gene in D. melanogaster resulted in over 50 % of the larvae in lethality during the second pupation; only 5 % of the flies reached the adult stage. There were unreparable damages and erratic behavior during the molt (Meng et al. 2002).
PBAN stimulates via the PBAN receptor , a heptahelical GPCR ; calcium influx and cAMP increase in cells of the pheromone gland . Thus the acetyl CoA carboxylase activity is enhanced in some species, and in other ones the reduction of fatty acids to aldehydes or alcohols is stimulated, which in turn induced pheromone biosynthesis and release (Tillman et al. 1999). The pyrokinin-1 receptor of the fruit fly is a heptahelical GPCR as well (Cazzamali et al. 2005).
5.3.2 Orcokinins
Fact sheet 5.10: Orcokinins
Sequence
Fig. 5.10.
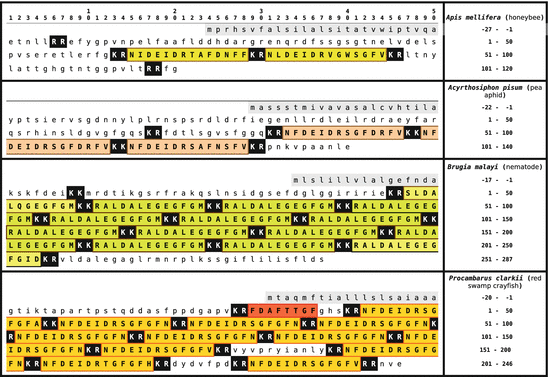

Fig. 5.10
Primary sequences of orcokinins from the arthropods (honeybee) , pea aphid (Acyrthosiphon pisum), of the nematode Brugia malayi, and the red swamp crayfish Procambarus clarkii. Signal peptides are on gray background, orcokinins are boxed and on colored background. Dibasic peptide motifs are shown white on black (Origin: GenBank P85832, XP_001947462, EDP37605, Q9NL82)
Synthesis and target
Orcokinins are neuropeptides, for example, from the stomatogastric ganglion; they act as neurotransmitters and hormones.
Function
Orcokinins contract the gut muscles and regulate the rhythm in the pylorus. They participate in the circadian regulation of motor activity.
Receptor
(not yet known).
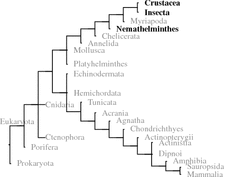
5.3.2.1 Introduction
From O. limosus 14 Stangier et al. (1992) reported a gut-active neuropeptide: orcokinin. Later they identified similar peptides in other crabs. Orcokinins have now been found in crustaceans, insects, and nematodes; potential mussel or snail orcokinins are not specified as sequences in GenBank.
5.3.2.2 Biochemistry and Structure
In some crustaceans and nematodes there are 10 and more identical or very similar neuropeptides on the same orcokinin precursor to be cleaved and released by prohormone convertase 1 or prohormone convertase 2 (KR or KK motif). In insects there are only few orcokinin s on the precursor, however, the N-terminal propeptide sequences are longer (Fig. 5.10). Bees only need PC1 ; the pea aphid (Acyrthosiphon pisum ) has to use PC1 and PC2 for orcokinin release (Fig. 5.10).
The red boxed FDAFTTGFamide peptide in Fig. 5.10 from the red swamp crayfish (P. clarkii 15 ) was first isolated and analyzed as orcomyotropin from O. limosus . That precursor of O. limosus has not been sequenced.
Until now no orcokinin receptor has been described. Because in C. elegans orcokinins and all the GPCR are known due to total genome sequencing, an orcokinin receptor might most probably be found in this species.
5.3.2.3 Physiology
The evolutionary conservation of the neuropeptide structure within the prepropolypeptide in nematodes and crustaceans may point to essential functions of orcokinins. Orcokinin from O. limosus acts on gut contractions: contraction amplitude and frequency are enhanced. Orcokinin forming neuron s were detected in the stomatogastric ganglion and other ganglia. They exhibit stimulatory activity on rhythm and activity of the pylorus . Whether orcokinins act as neurotransmitters or in an endocrine fashion via the hemolymph is not yet decided. In the hemolymph constant concentrations of orcokinins were observed. On the other hand the middle gut is innervated by several orcokinin neurons.
Finally another orcokinin function in the circadian control of locomotor activity in cockroaches has recently been found. Injections of orcokinin into the accessory medulla of the lobus opticus resulted in stable phase shifts in locomotor activity (Hofer and Homberg 2006).
5.3.2.4 Phylogeny
To date, orcokinins were found in insects, crustaceans, and nematodes.
5.3.3 Leucokinins/Lymnokinins
Fact sheet 5.11: Leucokinins, Lymnokinins
Sequence:
Fig. 5.11.
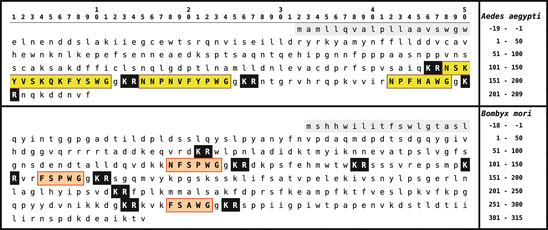

Fig. 5.11
Primary sequence of leucokinins from arthropods: silk moth (lower) and yellow-fever mosquito (upper panel). The signal peptide is shown on gray background, leucokinins are boxed and on yellow or red background. Dibasic peptide motifs are shown white on black (Origin: GenBank O02036, BAG50367)
Synthesis and target:
Leucokinins are brain neuropeptides that act via GPCR on muscle cells as well as on malpighian tubules.
Function:
Leucokinins stimulate gut musculature, but most of all they stimulate diuresis.
Receptor:
The lymnokinin receptor belongs to the heptahelical GPCR family.
5.3.3.1 Introduction
Leucokinins, locustakinin s, and lymnokinin s constitute the FxxWxamide neuropeptide family , found with similar structure in insects, molluscs, and nematodes. At first leucokinins were identified due to their gut contracting activity. In the meanwhile regulation of diuresis in malpighian tubules appears as the characteristic feature of leucokinins and related peptides.
5.3.3.2 Biochemistry and Structure
Leucokinins were originally isolated by Holman et al. (1986b,a) in the cockroach L. maderae 16 (Table 5.6). Precursor proteins have been identified in the silk moth , the yellow-fever mosquito , and D. melanogaster . The three peptide sequences of the yellow-fever mosquito precursor (Fig. 5.11) have been isolated. In the silk moth the precursor has been determined by genome sequencing; the kinins themselves are not yet proven. They might be N-terminally prolonged. The leucokinin of D. melanogaster , NSVVLGKKQR  HS
HS 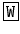 G-NH2 isolated by Baggerman et al. (2002), contains a dibasic peptide motif where prohormone convertase might cleave the precursor.
G-NH2 isolated by Baggerman et al. (2002), contains a dibasic peptide motif where prohormone convertase might cleave the precursor.
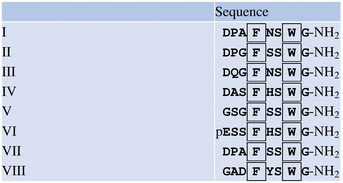
 HS
HS 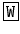 G-NH2 isolated by Baggerman et al. (2002), contains a dibasic peptide motif where prohormone convertase might cleave the precursor.
G-NH2 isolated by Baggerman et al. (2002), contains a dibasic peptide motif where prohormone convertase might cleave the precursor.Table 5.6
Kinins from the cockroach Leucophaea maderae

The lymnokinin from L. stagnalis 17 bears a C-terminal serine-amide: PS 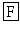 HS
HS 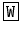 S-NH2 (Cox et al. 1997). In C. elegans a leucokinin-like precursor protein has been identified (GenBank AAM22049) containing twice the sequence qfyawag wherefrom a pE
S-NH2 (Cox et al. 1997). In C. elegans a leucokinin-like precursor protein has been identified (GenBank AAM22049) containing twice the sequence qfyawag wherefrom a pE 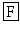 YA
YA 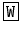 A-NH2 might result.
A-NH2 might result.
 HS
HS 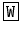 S-NH2 (Cox et al. 1997). In C. elegans a leucokinin-like precursor protein has been identified (GenBank AAM22049) containing twice the sequence qfyawag wherefrom a pE
S-NH2 (Cox et al. 1997). In C. elegans a leucokinin-like precursor protein has been identified (GenBank AAM22049) containing twice the sequence qfyawag wherefrom a pE  YA
YA 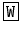 A-NH2 might result.
A-NH2 might result.The lymnokinin receptor has been first characterized by Cox et al. (1997). Other GPCR were later observed in insects: honeybees , fruit flies , and yellow fever mosquitoes . In ticks (i.e., in chelicerates) a very similar receptor was sequenced although a leucokinin has not been found in ticks. In the placozoan Trichoplax adhaerens , thus in an very basic metazoan species, a leucokinin-like receptor was sequenced together with the entire genome. Three A. aegyptii leucokinins (Fig. 5.11) were found to bind to the identical receptor, but with different affinities (Pietrantonio et al. 2005).
5.3.3.3 Physiology
Holman et al. (1986b) called the leucokinins originally cephalomyotropins , that is, peptides of the brain eliciting muscle contractions. When additional leucokinins were isolated, for example, in locusts, gut contractions served as the bioassay for testing of active fractions.
Leucokinins became important for insect research because they promote diuresis of sodium and potassium chloride. In blood-feeding insects, for example, A. aegyptii , a lot of salt and water has to be removed postprandially. This happens in the malpighian tubules. Therein leucokinins stimulate salt export . By inhibiting this export it might be possible to block reproduction of these dangerous disease vectors.
5.3.3.4 Phylogeny
Until now leucokinins and similar peptides of the FxxFxamide family have been found in molluscs, insects, and nematodes. A potential receptor might have already existed since the origin of metazoan evolution.
5.3.4 Tachykinin-Related Peptides (TRP)
Fact sheet 5.12: Tachykinin-related peptides
Sequence:
Fig. 5.12.

Fig. 5.12
Primary sequences of tachykinin-related peptide from L. maderae (LemTRP). The signal peptide is shown on gray background, the TRPs are boxed on yellow background. Dibasic peptide motifs are shown black on white. LemTRP-2 and LemTRP-3 have been found as long and short peptides that contain in their long form an intramolecular dibasic peptide motif (Origin: GenBank AAX11211; Nässel 1999; Predel et al. 2005)
Synthesis and target:
TRP is found in the brain, in several head ganglia, in the thorax and abdomen, as well as in neuroendocrine cells of the midgut.
Function:
TRP activates gut musculature and olfactory nerves.
Receptor:
The TRP receptor belongs to the rhodopsin family of heptahelical GPCR.
5.3.4.1 Introduction
In this book, tachykinins such as substance P have already been described among vertebrate hormones. These peptides bear a common FxGLM-NH2 motif . Invertebrate tachykinin-related peptides belong to the FxGxR-NH2 family (Nässel 1999; Review). They were found in several invertebrate phyla: molluscs, annelids, insects, and crustaceans.
5.3.4.2 Biochemistry and Structure
Up to 14 different tachykinin-related peptide sequences are present in the precursor gene of the TRP in cockroaches, for example, L. maderae , where the peptides could equally be identified by chemical analysis. However, in other species, only a few TRP peptides were found.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree