Outline
Microinvasive Carcinoma of the Cervix
Clinical Profile of Invasive Cancer
Glandular Tumors of the Cervix
Treatment of Early-Stage Disease
Radical Abdominal Hysterectomy With Lymphadenectomy
Indications for Postoperative Adjuvant Therapy
Nerve-Sparing Radical Hysterectomy
Sentinel Lymph Node Identification
Laparoscopic Radical Hysterectomy With Lymphadenectomy
Robotic-Assisted Laparoscopic Radical Hysterectomy With Lymphadenectomy
Fertility-Preserving Surgery for Early-Stage Tumors
Cervical Conization for Adenocarcinoma in Situ and Microinvasive Carcinoma
Vaginal Radical Trachelectomy With Laparoscopic Lymphadenectomy
Treatment of Locally Advanced Disease
Neuroendocrine and Other Uncommon Tumors of the Cervix
Glassy Cell Carcinoma, Carcinosarcoma, Lymphoma, and Melanoma
Survival Results and Prognostic Factors for Early-Stage and Locally Advanced Disease
Recurrent and Advanced Carcinoma of the Cervix
Key Points
- 1.
Conservative management of early staged cervical cancer in young women should be considered if childbearing is important to that patient.
- 2.
In many developing countries, cervical cancer is the leading cause of cancer death in young women.
- 3.
Adenocarcinoma of the cervix is increasing in frequency.
- 4.
Positron emission tomography is a very sensitive method for detecting the extent of cervical cancer.
- 5.
Adjuvant chemotherapy using cisplatin and concomitant radiation therapy is the standard of care for locally advanced cervical cancer.
General Observations
Anatomy
The cervix (Latin for “neck”) is a narrow, cylindrical segment of the uterus; it enters the vagina through the anterior vaginal wall and lies, in most cases, at a right angle to it. In the average patient, the cervix measures 2 to 4 cm in length and is contiguous with the inferior aspect of the uterine corpus. The point of juncture of the uterus and the cervix is known as the isthmus; this area is marked by slight constriction of the lumen. Anteriorly, the cervix is separated from the bladder by fatty tissue and is connected laterally to the broad ligament and parametrium (through which it obtains its blood supply). The lower intravaginal portion of the cervix, a free segment that projects into the vault of the vagina, is covered with mucous membrane. The cervix opens into the vaginal cavity through the external os. The cervical canal extends from the anatomic external os to the internal os, where it joins the uterine cavity. The histologic internal os is where there is a transition from endocervical to endometrial glands. The intravaginal portion of the cervix (portio vaginalis, exocervix) is covered with stratified squamous epithelium that is essentially identical to the epithelium of the vagina. The endocervical mucosa is arranged in branching folds (plicae palmatae) and is lined by cylindrical, columnar epithelium. The stroma of the cervix consists of connective tissue with stratified muscle fibers and elastic tissue. The elastic tissue is found primarily around the walls of the larger blood vessels.
The stratified squamous epithelium of the portio vaginalis is composed of several layers that are conventionally described as basal, parabasal, intermediate, and superficial. The basal layer consists of a single row of cells and rests on a thin basement membrane. This is the layer in which active mitosis occurs. The parabasal and intermediate layers together constitute the prickle-cell layer, which is analogous to the same layer in the epidermis. The superficial layer varies in thickness, depending on the degree of estrogen stimulation. It consists primarily of flattened cells that show an increasing degree of cytoplasmic acidophilia toward the surface. The thickness and the glycogen content of the epithelium increase after estrogen stimulation and account for the therapeutic effect of estrogens in atrophic vaginitis. The staining of glycogen in the normal epithelium of the portio vaginalis is the basis of the Schiller test.
Epidemiologic Studies
Clinical Profile
In the United States, the mortality from cervical cancer in 1945 was 15 of 100,000 women. This had declined to approximately 4.6 of 100,000 by 1986 and 3.4 of 100,000 by 1991. It is unclear whether the mortality rate from cervical cancer is falling as a result of cervical cytologic screening and intervention at the in situ stage or whether cervical screening has caused an increase in the proportion of early-stage cancer at diagnosis and registration. After therapy for invasive disease, adequate follow-up is the key to early detection of a recurrence ( Table 3.1 ). The yield of examinations such as intravenous pyelography (IVP), computed tomography (CT) scan, and chest radiograph in patients with initial early disease (stages I–IIa) is so low that many have discontinued their routine use.
| Year | Frequency | Examination |
|---|---|---|
| 1 | 3 months | Pelvic examination, Pap smear |
| 6 months | Chest radiography, CBC, BUN, creatinine | |
| 1 year | IVP or CT scan with contrast | |
| 2 | 4 months | Pelvic examination, Pap smear |
| 1 year | Chest radiography, CBC, BUN, creatinine, IVP or CT scan with contrast | |
| 3–5 | 6 months | Pelvic examination, Pap smear |
* Symptomatic patients should have appropriate examination where indicated.
West studied the age of registration and the age of death of women with cervical cancer in South Wales. He found that the observed age at death was very close to 59 years regardless of stage and age at diagnosis. Although the 5-year survival rate of women with localized (early-stage) cervical cancer was much higher than that of women with nonlocalized (late-stage) cancer, the women with localized cancer tended to be younger than those with advanced cancer. Calculations of expected age at death of the whole population suggest that more than half the advantage in survival rate shown by women with early-stage cancers is a result of the diagnosis of the former in younger women.
Christopherson and colleagues reported that the percentage of patients diagnosed as having stage I disease increased by 78% in the population studied from 1953 to 1965. The increase was most remarkable in younger women. The authors concluded that the major problem in cervical cancer control was the screening of older women. Older women had higher incidence rates; the percentage with stage I disease also decreased with each decade, reaching a low of 15% for those 70 years of age and older. These older women with cervical cancer are rarely screened and contribute heavily to the death rate. The initial advanced stage contributes to the patient population with advanced recurrent cervical cancer. These patients, therefore, deserve very close posttreatment observation in an effort to detect a recurrence in its earliest possible form.
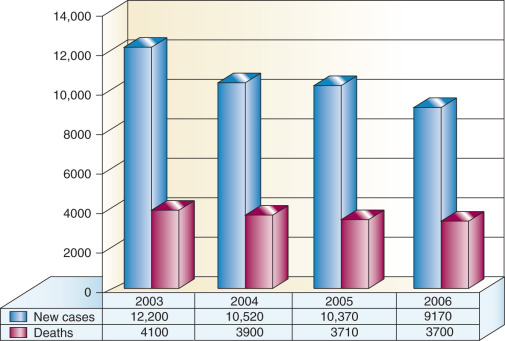
In 2011, there were 12,710 new cases of invasive cervical cancer and 4290 deaths from this disease in the United States. In fact, for the past decade, the annual incidence and mortality rates due to cervical cancer have been approximately 12,000 and 4,000, respectively. It is particularly distressing that more than one-third of women diagnosed will die from a disease that is largely preventable by vaccination and screening. There is no other human malignancy for which we have identified the causative agent, have successfully implemented excellent screening programs, and now have efficacious and tolerable prophylactic vaccination available ( Fig. 3.2 ). Oncogenic subtypes of the human papillomavirus (HPV) have been identified as the etiologic cause of cervical neoplasia ( Fig. 3.1 ). The power, consistency, and specificity of the association between subclinical HPV infection and cervical neoplasia raise the strong possibility that this relationship is causal. The biologic plausibility of this is supported by evidence that this sexually transmitted oncogenic virus often produces persistent asymptomatic infection of metaplastic epithelium in the cervical transformation zone.
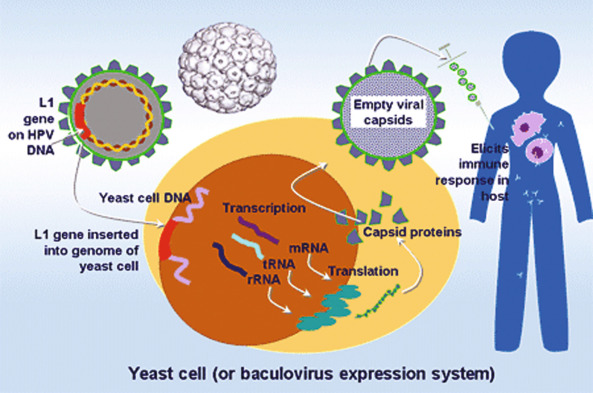
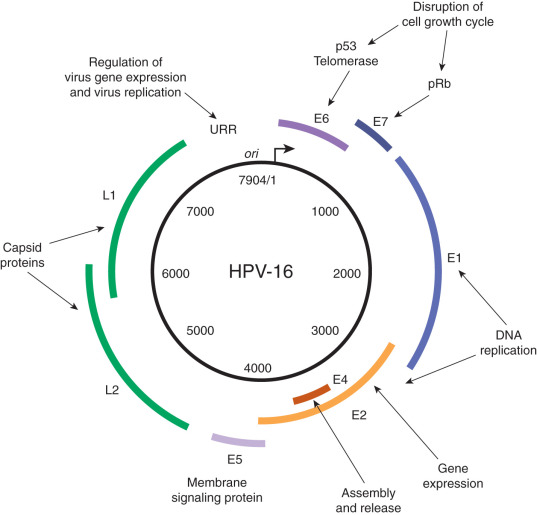
Epidemiologic surveillance studies performed in the United States during the past 2 decades have documented decreased incidence rates for invasive cervical cancer. Ethnic and racial disparities, however, still exist. In a Surveillance, Epidemiology and End Results (SEER) analysis of 13 US cancer registries containing cases from 1992 to 2003, Hispanic whites had the highest incidence rate of cervical cancer overall (24 per 100,000), squamous cell carcinoma (SCC) (18 per 100,000), and adenocarcinoma (5 per 10,000). Non-Hispanic whites had the lowest rates of cervical cancer overall (11 per 100,000) and SCC (7 per 100,000), and African Americans had the lowest rate of adenocarcinoma (2 per 100,000). In a recent study using data obtained from the Cancer in North America (CINA) deluxe 1995 to 2004 database created by the North American Association of Central Cancer Registries (NAACCR), African American and Hispanic US populations continue to have the highest rates of invasive cervical cancer compared with non-Hispanic whites. Variations in screening utilization and socioeconomic status are thought to account for the majority of the racial/ethnic disparities.
In November 2014, the nine-valent, HPV9 (providing protection against HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58), was approved by the US Food and Drug Administration (FDA) for the same indications as HPV-4 (ie, GARDASIL).
Microinvasive Carcinoma of the Cervix
The diagnosis and management of microinvasive carcinoma of the cervix remains controversial. The evolution and sometimes revolution concerning the diagnosis and management have occurred since Mestwerdt, in 1947, observed that invasive cervical cancer diagnosed only microscopically could be cured by nonradical surgery. During the past 3 decades, definitions and treatment plans have changed dramatically. It is hoped that most of these changes had occurred as new data became available and that changes were therefore logical. Much of the confusion can be related to the fact that the Federation of International Gynecologists and Obstetricians (FIGO) has changed the criteria for early-stage invasive carcinoma of the cervix since 1960. These changes were made as additional information in regard to this disease process became available. Other influences, however, also contributed to the confusion. Over the years, as many as 20 different definitions have been proposed, and as many as 27 terms have been applied to this entity. The recommended therapy has also changed, going from radical surgery with any invasion to being more conservative with various depths of invasion.
In 1971, FIGO designated stage Ia carcinoma of the cervix as cases of preclinical carcinoma. In 1973, the Society of Gynecologic Oncologists (SGO) accepted the following statement concerning the definition of microinvasive carcinoma of the cervix:
- 1.
Cases of intraepithelial carcinoma with questionable invasion should be regarded as intraepithelial carcinoma; and
- 2.
A microinvasive lesion should be defined as one in which a neoplastic epithelium invaded the stroma in one or more places to the depth of less than 3 mm below the base of the epithelium and in which lymphatic or vascular involvement is not demonstrated.
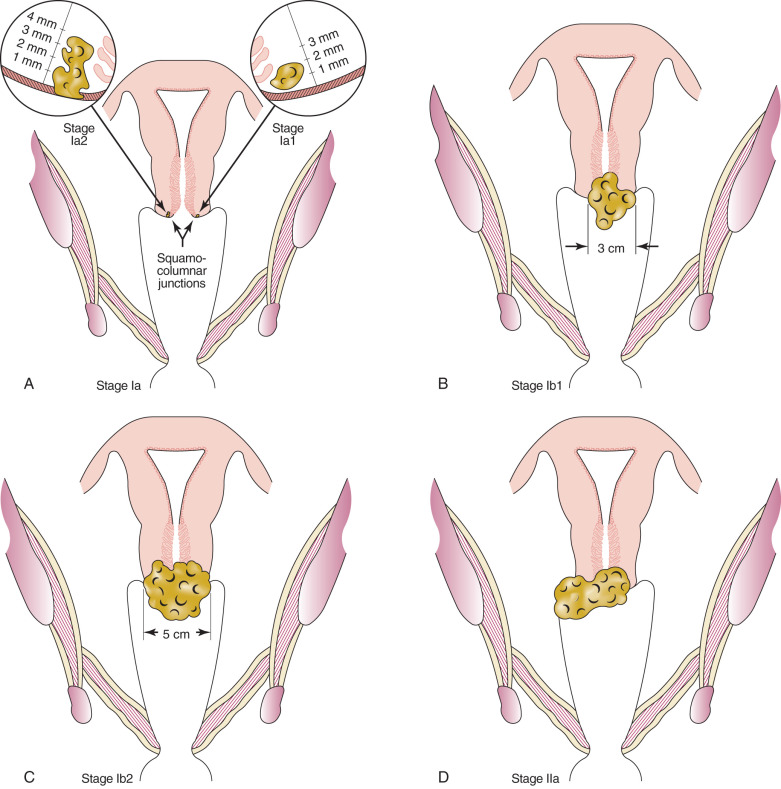
In 1985, for the first time, FIGO attempted to quantify the histologic definition of stage Ia carcinoma of the cervix. Stage Ia was defined as the earliest form of invasion in which minute foci of invasion are visible only microscopically. Stage Ia2 is a macroscopically measurable microcarcinoma that should not exceed 5 mm in depth and 7 mm in width. Vascular space involvement, either venous or lymphatic, should not alter staging. This definition has been criticized for several reasons. Although the upper limits of invasion for depth and width were stated, upper limits for measurement for stage Ia1 were not defined. It was therefore difficult to quantify patients in the two subgroups. Other areas of criticism were aimed at the fact that the FIGO definition could not be used as a guide for treatment, and the definition covered patients with vascular lymphatic channel involvement. These variations illustrate the problem with a specific definition.
In 1994, FIGO, in an attempt to better qualify the definition of microinvasive carcinoma of the cervix, adopted the following definition for microinvasive carcinoma of the cervix ( Table 3.2 ). Stage Ia1 cancers are those with stromal invasion up to 3 mm in depth and no greater than 7 mm. Stage Ia2 are when invasion is present at 3 to 5 mm in depth and no greater than 7 mm. Lymphatic vascular space involvement does not exclude a patient from this definition. The recurrence rate of patients in these two substages is probably no more than 1% to 2%. Survival of stage Ia1 approaches 99%, and stage Ia2 survival approaches 97% to 98%. This new definition allows further evaluation of what might be appropriate therapy for the different substages, particularly stage Ia2 cancers.
| Stage | Description |
|---|---|
| Ia | Cancer invasion identified only microscopically. All gross lesions, even with superficial invasion, are stage Ib cancers. Measured stromal invasion with maximum depth of 5 mm and no wider than 7 mm * |
| IaI | Measured invasion of stroma up to 3 mm |
| Ia2 | Measured invasion of stroma of 3–5 mm and no wider than 7 mm |
* The depth of invasion should not be larger than 5 mm taken from the base of the epithelium, either surface or glandular, from which it originates. Vascular space involvement, either venous or lymphatic, should not alter the staging.
Vascular space involvement was not excluded from the FIGO definition for several reasons. Pathologists disagree with regard to the reproducibility of this entity. At least in one study, the number of slides prepared from the cervix depended on the incidence of capillary-like space involvement. Shrinkage artifact can lead to an overdiagnosis, and verification has been suggested with special staining to verify true capillary-like space involvement. In one study in which immunoperoxidase staining with Ulex Europaeus agglutinin 1 lectin (UEAI) was used, 10 of 32 cases of vascular space involvement were excluded in which involvement was initially thought to exist. In a combined study of 1004 patients at three reference centers, Burghardt and colleagues observed that the frequency with which angiolymphatic space involvement was detected ranged from 9% in Munich to 23% in Erlangen and finally up to 43% in Graz.
As greater experience is obtained, the tendency has been toward conservative management involving conization of the cervix if fertility preservation is desired or simple hysterectomy for superficial invasion (0–3 mm) and even in some patients with 3–5 mm of invasion. In 1978, Lohe reported on 285 patients with early stromal invasion and 134 patients with microcarcinoma. He defined early stromal invasion as only isolated, variably shaped projections with true signs of infiltration present, whereas microcarcinomas’ true confluent carcinomatosis masses were present. Tumor length and depth were 10 mm and 5 mm. He stated that the three-dimensional definition of the size of the tumor is essential to the microscopic diagnosis of early stromal invasion and of microcarcinoma. In his series, 72% with early stromal invasion and 41% with microcarcinoma were treated with conservative surgery (conization or simple hysterectomy). After long-term follow-up, no patients with early stromal invasion died. Three patients with microcarcinomas have recurred and died. In a larger collected series of 435 patients with microcarcinoma, 24 (5.5%) had a recurrence of disease. Using his criteria, Lohe predicted <1% incidence of lymph node metastasis in microcarcinoma and essentially none in early stromal invasion. Boyce and colleagues have reported a large series with both “microscopic foci” of invasion (360 cases) and “occult invasion” (390 cases). Most (283) of the patients with microscopic foci invasion were treated with simple hysterectomy and only 14 with conization. After 5–15 years of follow-up, only one patient died of the disease. Most (262) of the patients with occult invasion received irradiation therapy. Most of these lesions were greater than 5 mm in depth, and all were characterized by confluent masses of neoplastic cells. Twenty-four of the 390 patients with occult invasion died of the disease in 5 years. Benedet and colleagues reported on 180 patients with microinvasion and occult invasive squamous cell carcinoma of the cervix who were examined by colposcopy during a 10-year period. Colposcopy led to the correct management in 90% of patients with occult invasive carcinoma and in 84% of patients with microinvasive cancer. Colposcopy appeared to be less sensitive in detecting microinvasive lesions than in detecting occult carcinoma. Atypical vessels indicative of early invasive cancer are often subtle and difficult to identify. They are best visualized when the cervix has been bathed in normal saline. In many cases, acetic acid can cover atypical vessels by enhancing white epithelium that obscures the vessels. Östör in Australia has made an extensive review on this subject of the literature published since 1976. As a pathologist, he has critically reviewed many of the parameters that have historically been suggested as important prognostic factors.
0–3 mm Invasion
Östör identified 3683 patients from the literature with <1 mm of invasion. Four patients had lymph node metastasis. With the incidence of lymph node metastasis in this group being essentially 0, it appears that lymphadenectomy has no place in this group of patients. There were 17 invasive recurrences and 16 cancer-related deaths. In several cases that resulted in death, patients refused further therapy or follow-up. Therefore, the death rate in this group of patients appears to be <0.1%, invasive recurrences were approximately 0.4% ( Table 3.3 ). Östör identified 1324 patients who had invasion of 1–3 mm. Of these, 333 definitely had lymphadenectomies with seven nodal metastases. At least two of these probably had >3 mm of stromal invasion. The incidence based on these figures suggests that lymph node metastasis would be approximately 1%. There were 26 invasive recurrences and 6 deaths. Some of these patients probably had >3 mm of invasion and also included some patients with “microinvasive adenocarcinoma”. In a review of reports over the last 15 years, most of which occurred within the last decade, Creasman and colleagues identified 1704 patients who had an invasion of 0–3 mm and 17 (1%) recurred. Only three (0.17%) died of their cancer. These recurrences were in patients with squamous lesions.
| Invasion Depth (mm) | No. of Patients | Invasion Recurrence | Died of Cancer | No. With CLSI | No. With Positive Nodes |
|---|---|---|---|---|---|
| 0–3 | 5007 | 35 (0.7%) | 10 (0.2%) | 182 (3.6%) | 8/666 (1.2%) |
| 3–5 | 674 | 25 (4%) | 13 (2%) | 124 (18.4%) | 14/221 (6.3%) |
The management of early invasive lesions (0–3 mm of invasion, Fig. 3.3A–C ) has generally been agreed on by the gynecologic oncology community. Patients with FIGO stage Ia1 and SGO criteria for microinvasion could be treated conservatively with simple hysterectomy, or if continued fertility is desired conization only, provided surgical margins are free of cancer. Most of the data in the literature concerning patients with 0–3 mm of invasion are based on conservative therapy, although some patients have had lymphadenectomies. From collected series, it would, therefore, appear that the role of vascular space involvement in this group of patients does not predict lymph node metastasis or recurrence. Although still in dispute, a growing number of investigators apparently do not use capillary-like space involvement as an exclusion criterion for this stage.
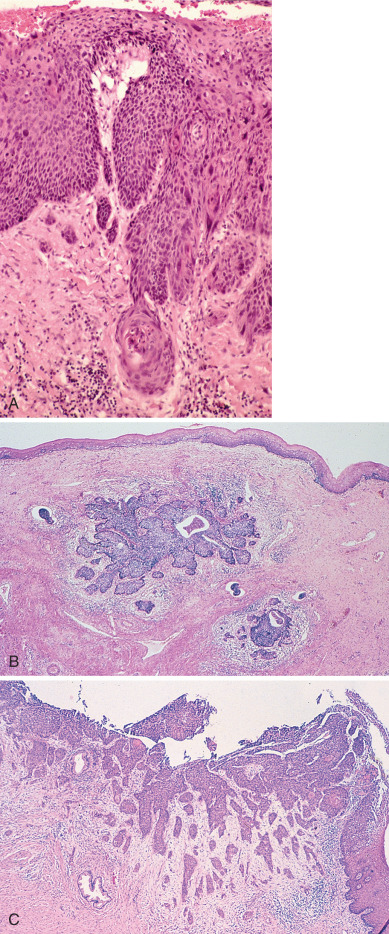
3–5 mm Invasion
Östör identified 674 patients in this category of which 221 definitely had lymphadenectomies, 6% had nodal metastasis. If most or all of the other patients had lymphadenectomy, which is possible, and the results of all of the nodes were negative, the figure would be close to 2%. Twentyfive (4%) patients developed an invasive recurrence, and 13 (2%) died of their disease. Twenty-three percent of the patients were said to have had capillary-like space involvement. Creasman identified from the literature 264 patients with 3–5 mm invasion who had lymphadenectomy in which vascular space involvement was specifically evaluated. In patients with vascular space involvement, 2 of 83 (2.4%) and 7 of 181 (3.8%) without vascular space involvement had lymph node metastasis. In the literature, 488 patients with 3–5 mm were identified and 15 (3%) developed a recurrence with 9 (1.8%) dying of cancer deaths. The recurrence and death rates in patients with 3–5 mm approaches those with 0–3 mm invasion. Most, but not all, of patients treated for 3–5 mm invasion were managed with radical hysterectomy and pelvic lymphadenectomy. However, there are patients in this category who have been managed only with conization. In the 3–5 mm range, vascular space status does not appear to correlate with lymph node metastasis. There is limited experience with patients with these clinical lesions, but patients with limited invasion of 3–5 mm have a much greater incidence of lymph node metastasis (7%), which should be treated as a stage Ib cancer.
The data on patients with 3–5 mm invasion still remain fairly limited. With a very low incidence of lymph node metastasis (2–3%), it seems reasonable to evaluate the role of more conservative therapy in this group of patients. This may be done after consultation with the pathologist and gynecologic oncologist. If one is concerned about the 3% incidence of lymph node metastasis, these nodes can be evaluated with laparoscopy or retroperitoneal lymphadenectomy. If there is no evidence of extrauterine disease via these procedures, simple hysterectomy or even conization may be reasonable treatments. Twenty years ago, it was not unusual for investigators to suggest radical hysterectomy and pelvic lymphadenectomy for any degree of stromal invasion. With increased experience, more conservative therapy was found to be just as efficacious as radical therapy. It appears that we are now in the same arena for 3–5 mm today as we were for 0–3 mm two decades ago. Although vascular space involvement does not appear to predict lymph node metastasis with 3–5 mm invasion, anecdotal reports in the literature have suggested that recurrences are higher in patients with capillary-like space involvement but with no metastasis in the lymph nodes.
It may be of significance that with progressively greater depth of stromal invasion, there is an increased risk of angiolymphatic space involvement. In point of fact, Fuller et al from the Memorial Sloan-Kettering Cancer Center detected a linear relationship between depth of cervical stromal invasion and the presence of angiolymphatic invasion. Although vascular space involvement was predictive of recurrence to the same degree it was predictive of nodal metastases, when stratified for the latter, vascular invasion lost its adverse prognostic effects. Östör noted in his review that 496 (14%) of 3597 patients who had capillarylike space involvement did not have an invasive recurrence. Morice et al retrospectively studied the clinicopathologic features of 193 patients who underwent radical surgery and lymphadenectomy for early stage cervical carcinoma, and found that both lymphovascular and nodal status were prognostic factors upon multivariate analysis. It was noteworthy that among a subgroup of 89 patients with a small tumor (<2 cm) and absence of nodal or isthmic involvement, that the overall survival was significantly correlated with the presence of lymphovascular space involvement. Recently, in an analysis of 93 patients who had undergone resection of early stage lesions, Memarzadeh et al demonstrated that the presence of lymphovascular space invasion in the parametria was an independent predictor of metastases in the pelvic ( P < 0.001) and para-aortic ( P < 0.05) lymphatic chains, an altogether not surprising finding. Hopefully, the role of lymphovascular space involvement will be further clarified and possibly even resolved in the future as more data are accumulated.
The Gynecologic Oncology Group (GOG) reported 50 patients with 3–5 mm invasion in the conization specimen. All underwent a radical hysterectomy and pelvic lymphadenectomy. The uterine specimen showed no evidence of residual disease. There were 23% with lymphatic space involvement (LSI), but none of the patients had positive nodes and there have been no recurrences. (Note that the term LSI is used interchangeably with vascular space involvement [VSI].)
In a report from Japan, 402 patients were identified with 0–5 mm invasion. These authors noted that 72 (18%), although had depth of invasion satisfying the FIGO definition, had >7 mm of horizontal spread. As the depth of invasion increased, there was a greater possibility that horizontal spread was >7 mm (6% stage Ia1 and 61% for stage Ia2). Lymph node metastasis was 1.4% for true Ia1 and 3.4% for Ia2 cancers. For those with >7 mm spread, lymph node metastasis was 7.4%. LSI was 18% in those >7 mm compared to 3.6% if <7 mm. Four patients had a recurrence, but three recurrences occurred in those with >7 mm lateral spread. Other authors have noted recurrences and deaths in patients with 0–5 mm invasion; however, when lateral spread was evaluated, it was >7 mm. Tumor volume is an important prediction for metastasis, recurrence, and cancer-related deaths. Now that there are quantifiable perimeters for stage Ia cancers, more precise data can be evaluated with regard to risk and appropriate therapy for stage Ia2 can be considered.
Conservative management for stage Ia2 carcinoma of the cervix does not seem unreasonable in light of the fact that some investigators have suggested that conservative therapy might be feasible for patients with “small stage Ib” cervical cancers. Girardi and colleagues from Graz reported on a series of 69 patients with small stage Ib carcinomas of the cervix. Treatment consisted of conization or simple hysterectomy in 27, radical hysterectomy with lymphadenectomy in 25, radical vaginal hysterectomy in 13, and conization followed by radiotherapy in 4. No patient developed a recurrence during the ensuing 2–35 years. Two of the 25 patients with lymphadenectomy had one positive lymph node each. This study comes from a group of investigators who over the years have meticulously evaluated pathologic specimens and traditionally have been surgically aggressive. Östör reviewed the literature since 1976 with regard to conization as definitive therapy for stage Ia cancers. He identified 655 patients with 0–5 mm invasion treated with conization, which resulted in 12 invasive recurrences and one death.
Clinical Profile of Invasive Cancer
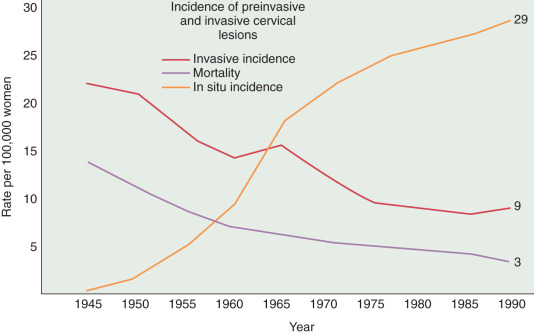
A substantial and well-publicized screening program is needed to make the public and the profession more aware of cervical cancer as the possible cause of even minimal gynecologic symptoms. All public education should emphasize the prevention and cure of cancer, and a more optimistic attitude would help motivate patients and physicians to seek appropriate action. The need for early diagnosis rests on the incontrovertible fact that definite cure, in actuarial terms, is readily achieved when cervical cancer is minimal—but almost impossible if the tumor is given time to grow and spread to the pelvic wall or into adjacent structures such as the bladder and rectum. The gradient of percentage curability from early invasive cancer to late, grossly invasive disease is such a steep one that even a moderate reduction in tumor size could not fail to create a substantial improvement in curability. It is true, of course, as with other cancers, that some carcinomas of the cervix grow more rapidly than others. The basis for this difference in growth rate is still beyond our knowledge, but it is not beyond our capability to prevent unnecessary growing time. Even the relatively slow-growing malignancy, if given enough time, will become incurable, and the most rapid-growing tumor, if diagnosed while of still moderate dimension, is definitely curable. The earlier that most tumors are detected and treated, the better will be the chance of cure. A Pap smear from a patient with early invasive SCC illustrates a typical multinucleated “tadpole” cell ( Fig. 3.4 ). Cytology and colposcopy are valuable tools in the eradication of cervical cancer. Every opportunity should be taken to disseminate modern concepts of cancer control to schools of nursing and other paramedical organizations because there is still a need for a more coordinated effort in these fields. The burden should not be left with physicians alone. The frequency with which invasive cervical cancer occurs in the United States is unknown, but the best incidence data indicate a rate of approximately 8 to 10 per 100,000/year ( Fig. 3.5 ). The incidence and mortality rates in the United States have been slowly declining ( Fig. 3.6 ). The occurrence of cervical cancer is apparently less frequent in Norway and Sweden than in the United States. However, in the underdeveloped areas of the world, the frequency of cervical cancer is more noteworthy, relative to the overall cancer problem, especially compared with that in the United States (see Table 3.2 ) and Western Europe.
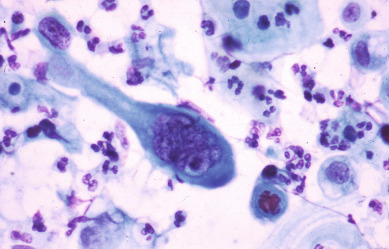
In many South American and Asian countries, cervical cancer accounts for the largest percentage of cancer deaths in women. One wonders whether nutritional deficiencies in these underdeveloped nations play a role in the etiology of cervical cancer. Orr and colleagues reported that abnormal vitamin levels were more commonly present in patients with cervical cancer. When compared with control values, levels of plasma folate, beta-carotene, and vitamin C were significantly lower in patients with cervical cancer. Personal cigarette smoking and exposure to passive smoke as risk factors for cervical carcinoma have been examined in case-control studies. Personal cigarette smoking increases the risk of cervical cancer after adjustment for age, educational level, church attendance, and sexual activity. The adjusted risk estimate associated with being a current smoker was 3.42; for having smoked for 5 or more pack-years, it was 2.81; and for having smoked at least 100 lifetime cigarettes, it was 2.21. The adjusted risk estimate associated with passive smoke exposure for 3 hours or more per day was 2.96. This study, reported by Slattery and colleagues in 1989, has been reinforced by others, confirming a strong association of smoking and increased risk of SCC of the cervix.
Some studies suggest that cancer of the cervix is more frequent among oral contraceptive users; however, these studies may be influenced by confounding factors such as early onset of sexual activity after puberty, multiple sexual partners, and previous history of sexually transmitted diseases. Ursin and colleagues reported a twofold greater risk of adenocarcinoma of the cervix, especially among those who used oral contraceptives for 12 years or more.
Because of the cervix’s sensitivity to hormonal influences, it may be considered biologically plausible that oral contraceptives could induce or promote cervical carcinoma. Piver reviewed a large number of early investigations of this issue and failed to show a consistent association. Moreover, these data are based on exposure to oral contraceptive preparations that contained high doses of estrogen and progestin and are no longer available.
In most large series, approximately 75% to 80% of malignant lesions of the cervix are squamous cell, but other lesions are possible ( Table 3.4 ). Most information regarding etiology and epidemiology is pertinent only to the more common squamous cell lesions.
| Epithelial Tumors | ||
|---|---|---|
| Nonglandular | Glandular | Other, Including Mixed |
|
|
|
| Nonepithelial Tumors | ||
| Mesenchymal Tumors | Germ Cell Tumors | Miscellaneous |
|
|
|
The greatest risk for cervical cancer is not ever having a Pap test or obtaining one infrequently. Everywhere in the world where the incidence of cervical cancer and its death rates have decreased, an active screening program is present. The older patients have a higher incidence of cervical cancer, at least in the United States, and these women have the most infrequent Pap smear screening.
Symptoms
A typical patient with clinically obvious cervical cancer is a multiparous woman between 45 and 55 years who married and delivered her first child at an early age, usually before 20 years of age. Probably the first symptom of early cancer of the cervix is a thin, watery, blood-tinged vaginal discharge that frequently goes unrecognized by the patient. The classic symptom is intermittent, painless metrorrhagia or spotting only postcoitally or after douching, although this is not the most common symptom. As the malignancy enlarges, the bleeding episodes become heavier and more frequent, and they last longer. The patient may also describe what seems to her to be an increase in the amount and duration of her regular menstrual flow; ultimately, the bleeding becomes continuous. In postmenopausal women, the bleeding is more likely to prompt early medical attention.
Late symptoms or indicators of more advanced disease include the development of pain referred to the flank or leg, which is usually secondary to the involvement of the ureters, pelvic wall, or sciatic nerve routes. Many patients complain of dysuria, hematuria, rectal bleeding, or obstipation resulting from bladder or rectal invasion. Distant metastasis and persistent edema of one or both lower extremities as a result of lymphatic and venous blockage by extensive pelvic wall disease are late manifestations of primary disease and frequent manifestations of recurrent disease. Massive hemorrhage and development of uremia with profound inanition may also occur and occasionally be the initial presenting symptom.
Gross Appearance
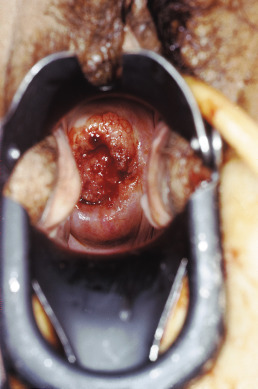
The gross clinical appearance of carcinoma of the cervix varies considerably and depends on the regional mode of involvement and the nature of the particular lesion’s growth pattern. Three categories of gross lesions have traditionally been described. The most common is the exophytic lesion, which usually arises on the ectocervix and often grows to form a large, friable, polypoid mass that can bleed profusely. These exophytic lesions sometimes arise within the endocervical canal and distend the cervix and the endocervical canal, creating the so-called barrel-shaped lesion. A second type of cervical carcinoma is created by an infiltrating tumor that tends to show little visible ulceration or exophytic mass but is initially seen as a stone-hard cervix that regresses slowly with radiation therapy. A third category of lesion is the ulcerative tumor ( Fig. 3.7 ), which usually erodes a portion of the cervix, often replacing the cervix and a portion of the upper vaginal vault with a large crater associated with local infection and seropurulent discharge.
Routes of Spread
The main routes of spread of carcinoma of the cervix are as follows:
- 1.
Into the vaginal mucosa, extending microscopically down beyond visible or palpable disease
- 2.
Into the myometrium of the lower uterine segment and corpus, particularly with lesions originating in the endocervix
- 3.
Into the paracervical lymphatics and from there to the most commonly involved lymph nodes (ie, the obturator, hypogastric, and external iliac nodes)
- 4.
Direct extension into adjacent structures or parametria, which may reach to the obturator fascia and the wall of the true pelvis. Extension of the disease to involve the bladder or rectum can result, with or without the occurrence of a vesicovaginal or rectovaginal fistula.
The prevalence of lymph node disease correlated well with the stage of the malignancy in several anatomic studies. Lymph node involvement in stage I is between 15% and 20%; in stage II, it is between 25% and 40%; and in stage III, it is assumed that at least 50% have positive nodes. Variations are sometimes seen with different material. The best study of lymph node involvement in cervical cancer was done by Henriksen ( Fig. 3.8 ). The nodal groups described by Henriksen follow.
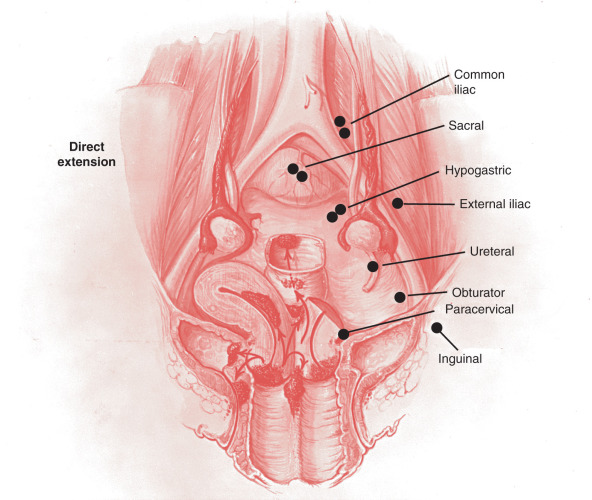
Primary Group
- 1.
The parametrial nodes, which are the small lymph nodes traversing the parametria
- 2.
The paracervical or ureteral nodes, located above the uterine artery where it crosses the ureter
- 3.
The obturator or hypogastric nodes surrounding the obturator vessels and nerves
- 4.
The hypogastric nodes, which course along the hypogastric vein near its junction with the external iliac vein
- 5.
The external iliac nodes, which are a group of six to eight nodes that tend to be uniformly larger than the nodes of the other iliac groups
- 6.
The sacral nodes, which were originally included in the secondary group
Secondary Group
- 1.
The common iliac nodes
- 2.
The inguinal nodes, which consist of the deep and superficial femoral lymph nodes
- 3.
The periaortic nodes
In his autopsy studies, Henriksen plotted the percentage of nodal involvement for treated and untreated patients ( Figs. 3.9 and 3.10 ). Distribution is, as one would expect, with a greater number of involved nodes found in the region of the cervix than in distant metastases. Although the series was an autopsy study, Henriksen found that only 27% had metastasis above the aortic chain. Cervical cancer kills by local extension, with ureteral obstruction in a high percentage of patients.
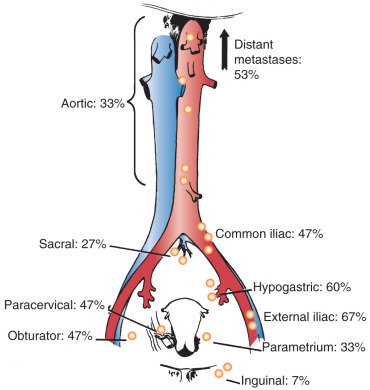
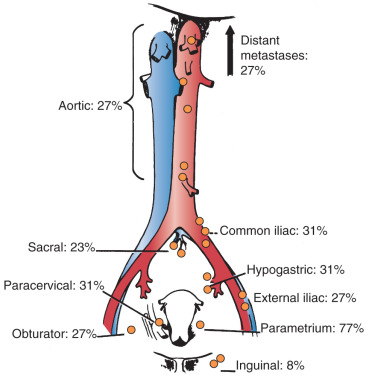
In 1980, the Gynecologic Oncology Group (GOG) reported the results of a series of 545 patients with cancer of the cervix who were surgically staged within their institutions. This study was prompted because traditional ports of radiation therapy were destined to treatment failure when the disease extended to the periaortic nodes ( Fig. 3.11 ). They found periaortic node involvement in 18.2% of patients with stage IIa disease and up to 33.3% in patients with stage IVa disease. Piver correlated the size of the cervical lesion with the incidence of lymph node metastasis in stage I disease ( Table 3.5 ).
| Site (cm) | Patients | Patients With Metastasis | Patients (%) |
|---|---|---|---|
| ≤1 | 22 | 4 | 18.1 |
| 2-3 | 72 | 16 | 22.1 |
| 4-5 | 45 | 16 | 35.5 |
| ≥6 | 6 | 3 | 50 |
| Total | 145 | 39 | 26.9 |
When clinical staging was compared with surgical staging, inaccuracies were found of the magnitude of a 22.9% misstaged occurrence in stage IIb disease and a 64.4% misstaged occurrence in stage IIIb disease. These data raise the question of whether knowing that disease has spread to the periaortic area enables the clinician to institute therapeutic modalities that can result in increased salvage. In other words, does the treatment of patients with spread of disease beyond the pelvis result in more cures? Berman and colleagues, reporting the GOG experience with staging laparotomy, indicated that 20% of 436 patients (stages IIb–IVa) were found to have metastatic disease to periaortic nodes. He also reported that 25% of these patients, or 5% of those surgically staged, demonstrated a 3-year, disease-free survival. Most of the patients with known periaortic node involvement received extended postoperative field irradiation.
Cumulative results from many studies utilizing lymphadenectomy in the surgical staging of cervix cancer have shown increased frequency of positive pelvic nodes, as shown in Table 3.6 .
| Clinical Stage | Positive Pelvic Nodes | Positive Periaortic Nodes |
|---|---|---|
| I | 15.4 | 6.3 |
| II | 28.6 | 16.5 |
| III | 47.0 | 8.6 |
Glandular Tumors of the Cervix
Approximately 75% to 80% of cervical cancers are SCCs, and most of the remaining cases are adenocarcinomas. There appears to be an increase in the frequency of cervical adenocarcinomas, but this may be a result of the decrease in the incidence of invasive squamous cell lesions. With respect to histopathology, a SEER population study conducted on cases registered from 1973 to 2002 noted increasing numbers of adenocarcinomas despite a general decline, suggesting the inefficiency of conventional screening for these tumors. Adenocarcinoma arises from the endocervical mucous-producing gland cells, and because of its origin within the cervix, it may be present for a considerable time before it becomes clinically evident. These lesions are characteristically bulky neoplasms that expand the cervical canal and create the so-called barrel-shaped lesions of the cervix. The spread pattern of these lesions is similar to that of SCC, with direct extension accompanied by metastases to regional pelvic nodes as the primary routes of dissemination. Local recurrence is more common in these lesions, and this has resulted in the commonly held belief that they are more radioresistant than are their squamous counterpart. It seems more likely, however, that the bulky, expansive nature of these endocervical lesions, rather than a differential in radiosensitivity, accounts for the local recurrence. Two controversial issues continue with regard to management of adenocarcinoma of the cervix. First, does this cell type carry a worse prognosis than squamous or adenosquamous cell types? Second, for early-stage disease, which therapy (radical surgery, radiation, or combined treatment) is superior?
Most studies suggest no difference in survival when adenocarcinomas are compared with SCCs after correction for stage. The 1998 FIGO Annual Report, which reported more than 10,000 SCCs and 1138 adenocarcinomas using multivariant analysis, noted no difference in survival in stage I cancers. In a study by Chen and associates of 302 adenocarcinomas, it was noted that in early stages, multivariant analysis noted better survival in patients treated with radical surgery compared with those treated with radiation therapy.
Kjorstad and Bond investigated the metastatic potential and patterns of dissemination in 150 patients with stage Ib adenocarcinoma of the cervix treated from 1956 to 1977. All patients were treated with a combination of intracavitary radium followed by radical hysterectomy with pelvic lymph node dissection. The incidence of pelvic metastases and distant recurrences and the survival rates were the same as those given in previously published reports for SCC treated in the same manner. In one respect, the adenocarcinomas showed a significant difference from the squamous cell cancers. The incidence of residual tumor in the hysterectomy specimens after intracavitary treatment was much higher (30% vs. 11%). Kjorstad and Bond considered this a strong argument for surgical treatment of patients with early stages of adenocarcinoma of the cervix.
Moberg and colleagues reported on 251 patients at Radiumhemmet in Stockholm with adenocarcinoma of the uterine cervix. The 5-year survival rate was compared with that in the total of cervical epithelial malignancies, and the rate was lower in the adenocarcinoma cases, with respective crude 5-year survival rates of 84%, 50%, and 9% in stages I, II, and III, respectively. Combined treatment consisting of two intracavitary radium treatments with an interval of 3 weeks followed by a radical hysterectomy with pelvic lymphadenectomy done within 3 months gave improved 5-year survival in a nonrandomized series. Prempree and colleagues also suggested combined therapy for stage II lesions or for those larger than 4 cm.
A large series of 367 cases of adenocarcinoma of the cervix was reported by Eifel and associates. Their conclusions were that the central control of adenocarcinomas with radiation therapy is comparable to that achieved for SCCs of comparable bulk. They found no evidence that combined treatment (radiation therapy plus hysterectomy) improved local regional control or survival. In their study, radiation therapy alone was as effective a treatment for most patients with stage I disease. They noted, as others have, that patients with bulky stage I (>6 cm), stage II, or stage III disease, particularly with poorly differentiated lesions or evidence of nodal spread, had a very high rate of extrapelvic disease spread.
Eifel reported the results of 160 patients with adenocarcinoma of the cervix. Of these patients, 84 were treated with radiation therapy alone; 20 were treated with external and intracavitary radiation followed by hysterectomy, and 56 were treated with radical hysterectomy. Survival was strongly correlated with tumor size and grade. There was a 90% survival rate for lesions smaller than 3 cm. After 5 years, 45% of the patients treated with radical hysterectomy had a recurrence. These recurrences were strongly correlated with lymph or vascular space invasion, poorly differentiated lesions, and larger tumor size.
Chen and associates from Taiwan reviewed 3678 cases of cervical cancer treated between 1977 and 1994, of which 302 (8.5%) were adenocarcinoma. A higher proportion of cases with adenocarcinoma were of the lower stages and in the younger patient even within a given stage. Survival was better in all stages in patients with squamous compared with adenocarcinoma (81% vs. 76% in stage I, P = 0.0039). When surgery was primary therapy, there was no difference in survival in stage I (83% vs. 80.3% survival of squamous and adenocarcinoma, respectively). Survival with radiation therapy noted 71% vs. 49%, respectively ( P = 0.0039), in stage I. Survival decreased as age increased within a given stage.
The MD Anderson Hospital group compared 1538 patients with SCC with 229 patients with adenocarcinoma, all stage Ib and treated with radiation. In patients with tumors larger than 4 cm, multivariate analysis confirmed that patients with adenocarcinoma had a significantly poorer survival than did those with SCC (59% vs. 73%). In a study by the GOG, 813 stage Ia2 and Ib cancers were evaluated. All were treated with radical hysterectomy. There were 645 squamous, 104 adenocarcinoma, and 64 adenosquamous cancers. Radiation was given postoperatively to 16% squamous, 13% adenocarcinomas, and 20% of adenosquamous patients. After adjusting for multiple risk factors, survival was worst for adenosquamous cancer compared with squamous and adenocarcinoma (71.8%, 82.1%, and 88%, respectively). A similar finding was noted in a study from Taiwan in which 134 stage Ib or II cervical adenocarcinomas or adenosquamous cancers were compared with 757 similarly staged squamous carcinomas treated with radical hysterectomy. The overall survival (OS) rate was 72.2% for the former compared with 81.2% for the SCCs. The histology was an independent prognostic factor for recurrence-free survival and OS.
Staging
The staging of cancer of the cervix is a clinical appraisal, preferably confirmed with the patient under anesthesia; it cannot be changed later if findings at operation or subsequent treatment reveal further advancement of the disease.
International Federation of Gynecology and Obstetrics
International classification of cancer of the cervix according to FIGO was recently revised in 2009 ( Fig. 3.12 ):
| Stage | Description |
|---|---|
| 0 | Carcinoma in situ, intraepithelial carcinoma |
| I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded) |
| Ia | Invasive cancer identified only microscopically; all gross lesions, even with superficial invasion, are stage Ib cancers. Invasion is limited to measured stromal invasion with maximum depth of 5 mm and no wider than 7 mm |
| Ia1 | Measured invasion of stroma no greater than 3 mm in depth and no wider than 7 mm |
| Ia2 | Measured invasion of stroma greater than 3 mm and no greater than 5 mm and no wider than 7 mm |
| Stage | Description |
|---|---|
| Ib | Clinical lesions confined to the cervix or preclinical lesions greater than stage Ia |
| Ib1 | Clinical lesions no greater than 4 cm |
| Ib2 | Clinical lesions greater than 4 cm |
| II | Involvement of the vagina but not the lower third, or infiltration of the parametria but not out to the sidewall |
| IIa | Involvement of the vagina but no evidence of parametrial involvement |
| IIa1 | Clinically visible lesion no greater than 4 cm in greatest dimension |
| IIa2 | Clinically visible lesion greater than 4 cm in greatest dimension |
| IIb | Infiltration of the parametria but not out to the sidewall |
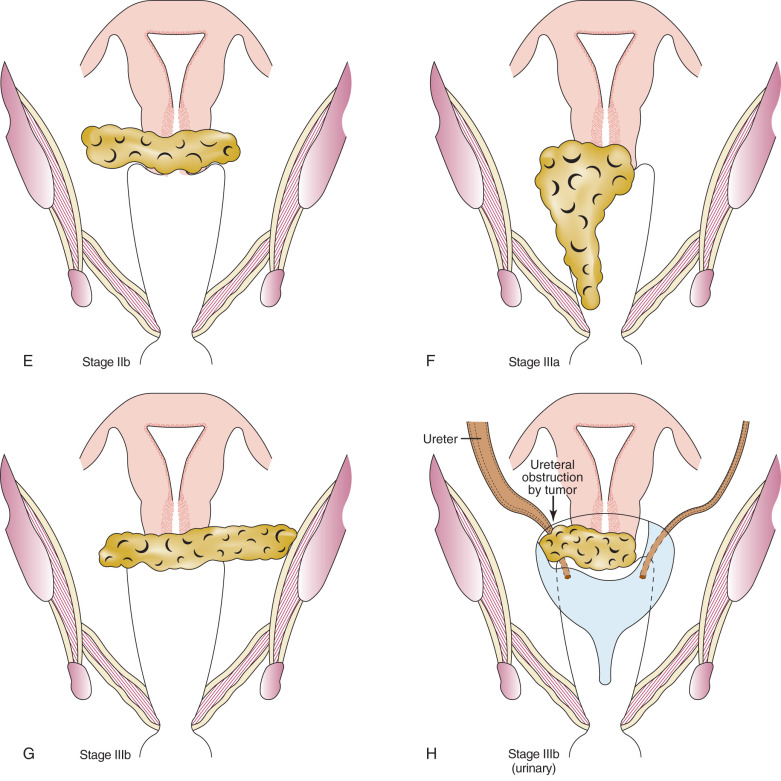
| III | Involvement of the lower third of the vagina or extension to the pelvic sidewall; all cases with a hydronephrosis or nonfunctioning kidney should be included unless they are known to be attributable to other causes |
| IIIa | Involvement of the lower third of the vagina but not out to the pelvic sidewall if the parametria are involved |
| IIIb | Extension onto the pelvic sidewall or hydronephrosis or nonfunctional kidney |
| IV | Extension outside the reproductive tract |
| IVa | Involvement of the mucosa of the bladder or rectum |
| IVb | Distant metastasis or disease outside the true pelvis |
In 2013, using the revised FIGO 2009 staging, Wagner et al. investigated the impact of size within FIGO stages I to IIIB using the 1988 to 2008 SEER Program database. The investigators evaluated a total of 18,649 cases and reported that the hazard ratios (HRs) were significantly worse for larger tumors on both univariate and multivariate modeling. The prognostic significance of the size subdivisions of 2 cm and 4 cm for stage 1, 4 cm for stage IIB, and 4 cm and 6 cm for stage IIIB were also maintained. These findings were believed to validate the changes to the FIGO system made in 2009.
The clinical evaluation of patients with cervical cancer is outlined in Table 3.7 . The following diagnostic aids are acceptable for determining a staging classification: physical examination, routine radiographs, colposcopy, cystoscopy, proctosigmoidoscopy, IVP, and barium studies of the lower colon and rectum. Other examinations, such as lymphography, CT scans, magnetic resonance imaging (MRI) examinations, arteriography, venography, laparoscopy, and hysteroscopy, are not recommended for staging because they are not uniformly available from institution to institution. It is important to emphasize that staging is a method of communicating between one institution and another. Probably more important, however, is that staging is a means of evaluating the treatment plans used within one institution. For these reasons, the method of staging should remain fairly constant. Staging does not define the treatment plan, and therapy can be tailored to the architecture of the malignancy in each patient.
| History | Review of Systems | General Physical Examination |
|---|---|---|
| Risk factors (STDs, smoking, OCPs, HIV), prior abnormal Pap tests, previous dysplasia and treatment | Abnormal vaginal bleeding or discharge; pelvic pain, flank pain, sciatica, hematuria, rectal bleeding, anorexia, weight loss, bone pain | Peripheral lymphadenopathy |
| Evaluation | Common procedures (FIGO) | Alternative procedures |
| Invasive cancer | Cervical biopsy | Histologic diagnosis required |
| Endocervical curettage | ||
| Cervical conization | ||
| Tumor size; involvement of the vagina, bladder, rectum and parametria | Pelvic examination under anesthesia | MRI pelvis preferred over CT |
| Anemia | Complete blood count | — |
| Renal failure | Serum chemistries | — |
| Hematuria | Urinalysis | — |
| Bladder involvement | Cystoscopy with biopsy and urine cytology | CT, MRI pelvis |
| Rectal infiltration | Proctoscopy with biopsy | CT, MRI pelvis; barium enema |
| Hydronephrosis | IVP | Renal ultrasonography; CT abdomen |
| Pulmonary metastases | Chest radiography | CT chest; PET scan |
| Retroperitoneal lymphadenopathy | — | Lymphangiogram, CT, MRI, PET scan |
Positron Emission Tomography
In 2005, the Centers for Medicare & Medicaid Services implemented coverage for 18-fluorodeoxyglucose positron emission tomography (FDG-PET) for patients with newly diagnosed and locally advanced cervical cancer undergoing pretreatment staging who have no extrapelvic metastases on conventional imaging studies ( Fig. 3.13 ). It should be noted that all imaging modalities are more specific than sensitive in detecting nodal metastases. The pooled sensitivity of PET in detecting pelvic nodal metastases in patients with untreated cervical cancer approaches 80% compared with MRI (≈70%) or CT (≈48%). It is important to recognize that the available studies are limited by low numbers of patients and wide confidence intervals (CIs).
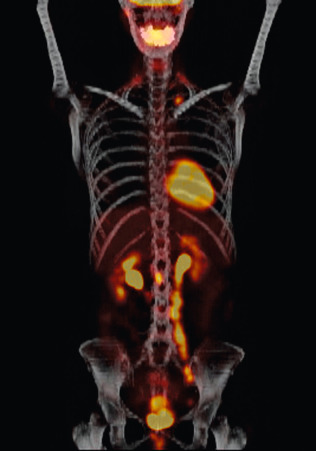
Grosu and colleagues from Munich analyzed the results of clinical studies on the integration of PET in target volume definition for lung, head and neck, GU, and brain tumors. FDG-PET had a significant impact on gross tumor volume and planning target volume delineation in lung cancer and was able to detect lymph node involvement and differentiate malignant tissue from atelectasis. In high-grade gliomas and meningiomas, methionine PET helped to differentiate tumor from normal tissue. Furthermore, the investigators suggest that FDG-PET seems to be particularly valuable in lymph node status definition in cervical cancer. With limited experience, several commentators have noted that FDG-PET may be superior to CT and MRI not only in the detection of lymph node metastases but also in the detection of unknown primary cancer and in the differentiation of viable tumor tissue after treatment. The accurate delineation of gross tumor volume suggests the potential for sparing of normal tissue. The imaging of hypoxia, cell proliferation, angiogenesis, apoptosis, and gene expression by new PET tracers such as choline and acetate may lead to the identification of different areas of a biologically heterogeneous tumor mass that can be individually targeted using intensity-modulated radiotherapy (IMRT). In addition, a biologic dose distribution can be generated permitting dose painting.
A 2007 meta-analysis of 41 studies was undertaken to compare the diagnostic performances of CT, MRI, and PET or PET/CT in patients for detection of metastatic lymph nodes in patients with cervical cancer. Whereas PET or PET/CT showed the highest pooled sensitivity (82%) and specificity (95%), CT showed 50% and 92% and MRI showed 56% and 91%, respectively ( Table 3.8 ). In a recent investigation of 83 women with FIGO stages IB1 to IIIB cervical cancer, F-18 fluorodeoxyglucose-avid pelvic lymph nodes (SUVPLN) was found to be a prognostic biomarker, predicting treatment response, pelvic recurrence risk, and disease-specific survival. Finally, a prospective validation study conducted between 2000 and 2009 enrolled 560 women who underwent pretreatment FDG-PET lymph node staging. Overall, 47% of patients had lymph node involvement by FDG-PET at diagnosis and within a stage, patients with PET-positive lymph nodes had significantly worse disease-specific survival than those with PET-negative lymph nodes ( P <0.001). The HRs for disease recurrence increased incrementally based on the most distant level of nodal disease: pelvic, 2.4 (95% confidence interval [CI], 1.63, 3.52); paraaortic, 5.88 (95% CI 3.8, 9.90); and supraclavicular, 30.27 (95% CI, 16.56, 55.34).
| n | FIGO Stages | Imaging Modality | Lymph Nodes | Sensitivity | |
|---|---|---|---|---|---|
| Sugawara et al. | 21 | IB-IVA | PET vs. CT | Overall | 0.86 (PET) 0.57 (CT) |
| Rose et al. | 32 | IIB-IVA | PET | PALN | 0.75 |
| PELN | 1.00 | ||||
| Yildirim et al. | 16 | IIB-IVA | PET | PALN | 0.5 |
| Grigsby et al. | 152 | IB-IV | PET | Overall | 0.67 |
| Narayan et al. | 7 | IB-IVB | PET | PELN | 0.80 |
| Yeh et al. | 42 | IB-IVA | PET | PALN | 0.83 |
| Lin et al. | 50 | IB-IVA | PET | PALN | 0.86 |
| Yen et al. | 135 | IB2-IVB + recurrence | PET | PELN | 0.88 |
| PALN | 0.95 | ||||
| Choi et al. | 22 | IB-IVA | PET-CT | PELN | 0.77 |
| Amit et al. | 75 | I-IV | PET-CT | PELN | 0.60 |
| Loft et al. | 119 | IB1-IVA | PET-CT | PELN | 0.96 |
| PALN | 1.00 |
Vanderperre et al. recently compared surgical staging with PET-CT, PET, and CT imaging of the paraortic lymph nodes in patients with locally advanced disease (ie, FIGO IB2-IVA). The 204 patients studied were divided into the following four groups: positive surgical staging with negative paraaortic imaging ( n = 16), negative surgical staging ( n = 172), positive imaging without surgical staging ( n = 20), and negative imaging without surgical staging ( n = 128). OS rates at 2 years (median follow-up 31 months) were 40%, 83%, 58%, and 69%, respectively ( P <0.001 for positive surgical staging and positive imaging without surgical staging vs. the other two groups). The authors noted that despite negative imaging, aortic nodal metastases were present among 8% of patients who underwent surgical staging, with OS being significantly influenced by the presence of paraaortic metastases.
Surgical Staging
Findings uncovered by fusion PET-CT or conventional MR and CT examinations can be used in the planning of therapy but should not influence the initial clinical staging of the lesion. Unfortunately, clinical staging is only of rough value in prognosis because disease distribution and extent are often included under one stage subheading. Clinical staging is enhanced with the liberal use of rectovaginal examinations ( Fig. 3.14 ) in that this type of pelvic examination allows more complete palpation of the parametria and cul-de-sac. The role of surgical assessment of lymph nodes with extraperitoneal, laparoscopic, or robotic lymphadenectomy is expanding. The ability to perform pelvic and paraaortic lymphadenectomy provides prognostic information, improves direction of radiation therapy (ie, extended-field radiation with positive paraaortic nodes) and may offer a therapeutic effect particularly in grossly involved lymph nodes. To date, no prospective data on surgical staging of cancers of the cervix exist to indicate a survival advantage to this approach.
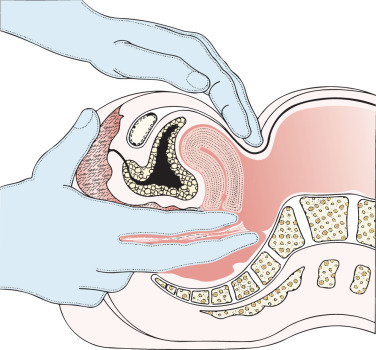
Some gynecologic oncologists believe that limited staging procedures are warranted on patients with advanced-stage cervical cancer to place patients on institutional or national group protocols. The status of paraaortic nodes should be known before treatment is initiated in such cases to plan appropriate modalities, such as the extent of the radiation field or concomitant chemotherapy. An extraperitoneal approach for removal of the periaortic nodes is preferred by many clinicians in an effort to reduce morbidity from the procedure. More advanced lesions have been investigated with a retroperitoneal lymphadenectomy to determine the extent of disease before planning radiotherapy fields ( Fig. 3.15 ). Fig. 3.16 illustrates one such approach. With the increased use of PET, it is expected that the indications for surgical staging in cervical cancer will decrease.
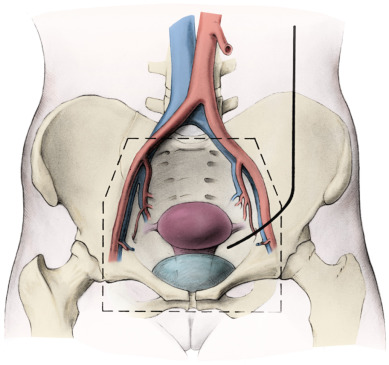
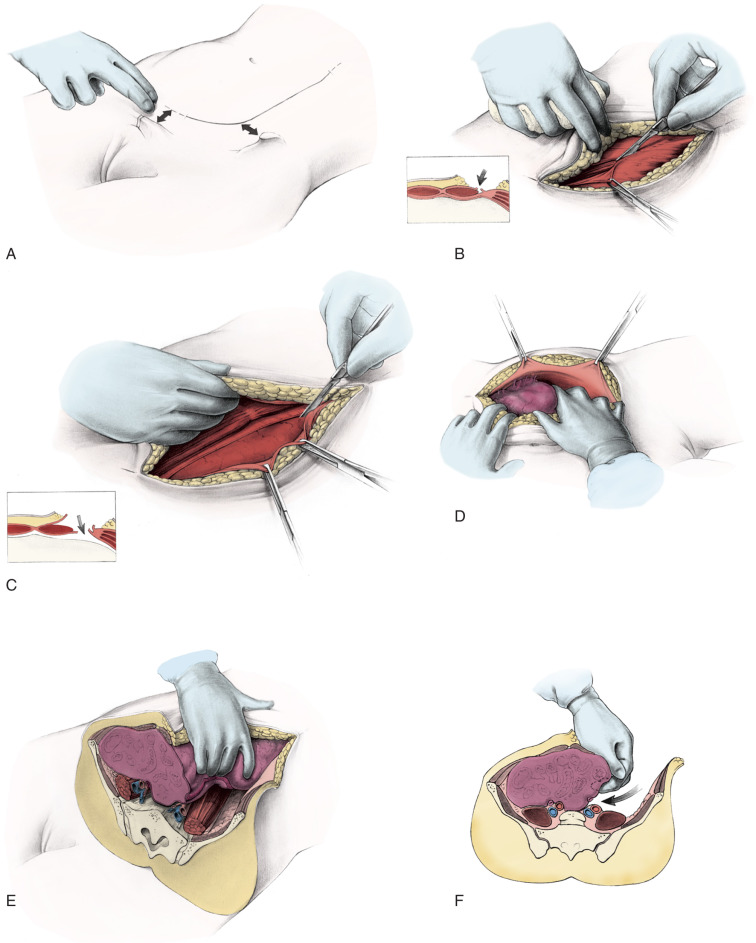
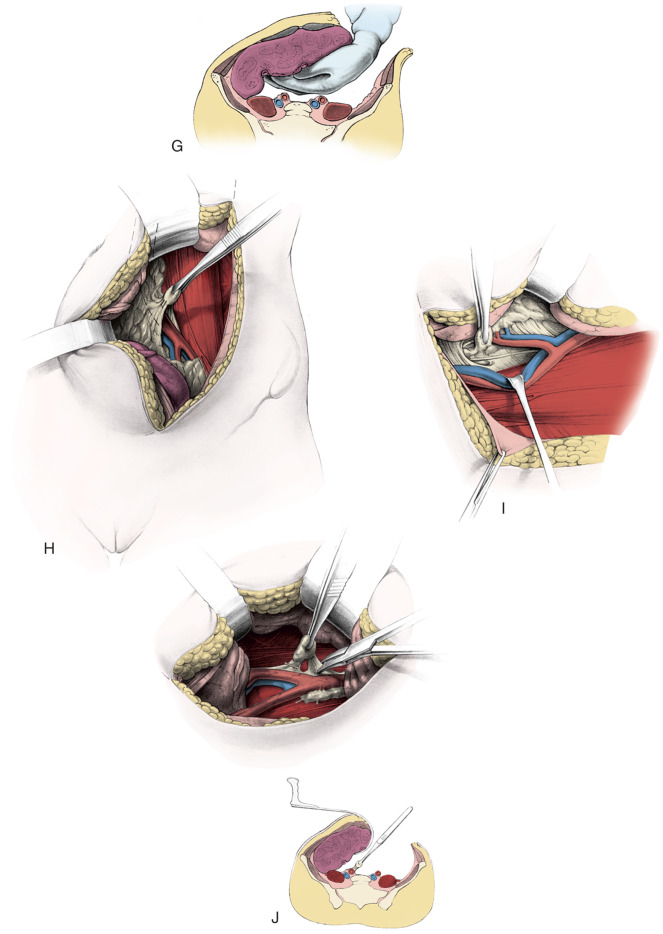
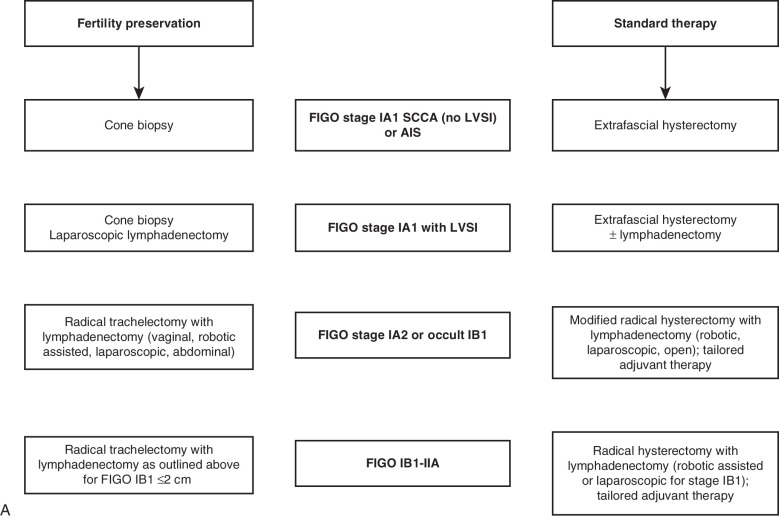
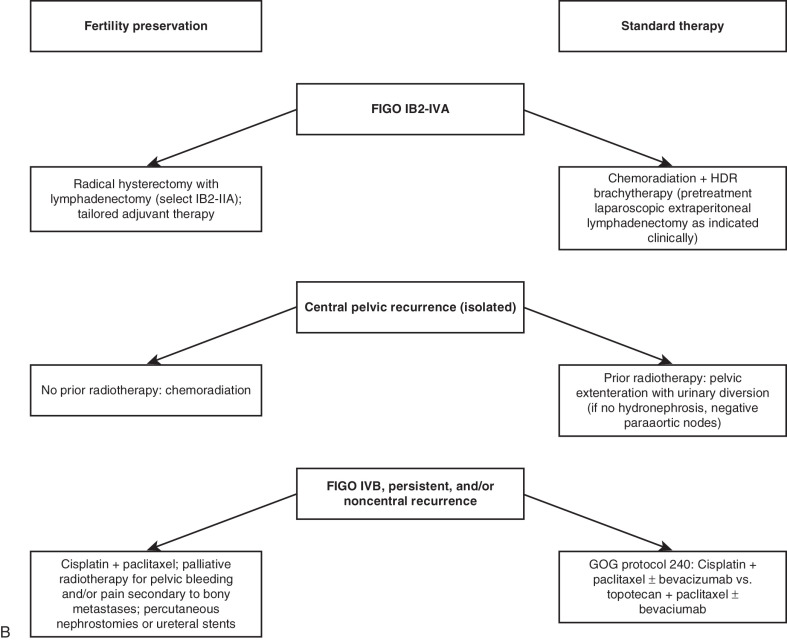
Treatment of Early-Stage Disease
After the diagnosis of invasive cervical cancer is established, the question is how to best treat the patient. Proposed management algorithms for early-stage disease, locally advanced malignancy, and disseminated tumors appear in Fig. 3.17 . Specific therapeutic measures are usually governed by the age and general health of the patient, by the extent of the cancer, and by the presence and nature of any complicating abnormalities. It is thus essential to carry out a complete and careful investigation of the patient (see Table 3.7 ), and then a joint decision regarding treatment should be made by the radiotherapist and gynecologic oncologist. The choice of treatment demands clinical judgment, but apart from the occasional patient for whom only symptomatic treatment may be best, this choice lies between surgery and radiotherapy (almost always given with cisplatin chemotherapy). In most institutions, the initial method of treatment for locally advanced disease is chemoradiation, both intracavitary and external radiographic therapy. The controversy between surgery and radiotherapy has existed for decades and essentially surrounds the treatment of stage I and stage IIa cervical cancer (see Fig. 3.17 ). For the most part, most patients with stages more advanced than stage I and stage IIa are treated with combination cisplatin and radiotherapy (see Fig. 3.17 ). The 5-year survival figures from two large series, one treated with radiotherapy alone and the other with surgery, are included here. Currie reported the results of 552 radical operations for cancer of the cervix:
| Stage | Patients |
|---|---|
| Preinvasive carcinoma in situ | 555 cases (99.9%) |
| Stage I | 189 cases (86.3%) |
| Stage IIa | 103 cases (75%) |
| Stage IIb | 78 cases (58.9%) |
| Other stages | 41 cases (34.1%) |
Some of these patients with positive nodes received postoperative radiotherapy.
In 1981, Zander and colleagues reported results of a 20-year cooperative study from Germany of 1092 patients with stages Ib and II cancer of the cervix treated with radical hysterectomy of the Meigs type and bilateral pelvic lymphadenopathy. Of the 1092 patients, 50.6% had surgery only, with a 5-year survival rate of 84.5% in stage Ib and 71.1% in stage II (most were stage IIa). This correlates well with the figures reported by Currie and Falk. The rest of the patients reported by Zander received postoperative whole-pelvis irradiation therapy. No significant difference could be observed in the survival rates of patients undergoing only surgery compared with those of patients undergoing adjuvant postoperative radiation. In fact, in 199 patients with lymph node involvement, the difference in survival rates of those undergoing only surgery and those undergoing additional postoperative radiation therapy was statistically insignificant.
Landoni and colleagues from the University of Milan conducted a prospective randomized trial in 243 patients comparing class II versus class III radical hysterectomy in stage IB to IIA cervical cancer. Although mean blood loss and transfusions were similar for both arms, mean operative time and late urologic morbidity were significantly lower in patients who underwent class II radical hysterectomy. The use of adjuvant radiotherapy was similar in both arms (54%, 55%). The recurrence rate (24%, 26%) and overall disease-free survival (75%, 73%) was not significantly different between the arms. Multivariate analysis confirmed that survival did not depend on the type of operation. This rare phase III surgical trial in early-stage cervical cancer has served as the platform from which our (non–phase III) experience in less radical surgery for early-stage disease is based (eg, radical trachelectomy and cervical conization for fertility preservation in the setting of invasive disease).
In general, in the early stages, comparable survival rates result from both treatment techniques. The advantage of radiotherapy is that it is applicable to almost all patients, but radical surgery of necessity excludes certain patients who are medically inoperable. The possible occurrence of immediate serious morbidity must be kept in mind when this treatment plan is selected. In many institutions, surgery for stage I and stage IIa disease is reserved for young patients in whom preservation of ovarian function is desired and improved vaginal preservation is expected. The modern operative mortality rate and the postoperative ureterovaginal fistula rate both have been reported to be less than 1%, making an objective decision for therapy even more difficult. Other reasons given for the selection of radical surgery over radiation include cervical cancer in pregnancy, concomitant inflammatory disease of the bowel, previous irradiation therapy for other disease, presence of pelvic inflammatory disease or an adnexal neoplasm along with the malignancy, and patient preference. Among the disadvantages of radiation therapy, one must consider the permanent injury to the tissues of the normal organ bed of the neoplasm and the possibility of second malignancies developing in this bed.
Radical Abdominal Hysterectomy With Lymphadenectomy
The use of radical hysterectomy in the United States was initiated by Joe V. Meigs at Harvard University in 1944, and shortly thereafter, the radical hysterectomy with pelvic lymphadenopathy was adopted by many clinics in the United States because of dissatisfaction with the limitations of radiotherapy. Some had found that many lesions were not radiosensitive, and some patients had metastatic disease in regional lymph nodes that were alleged to be radioresistant. Radiation injuries had been reported, and one of the overriding points in favor of surgery was that gynecologists were surgeons rather than radiotherapists and thus felt more comfortable with this treatment. At the time of the popularization of this procedure, modern techniques of surgery, anesthesia, antibiotics, and electrolyte balance had emerged, reducing the enormous morbidity that once attended major operative procedures in the abdomen.
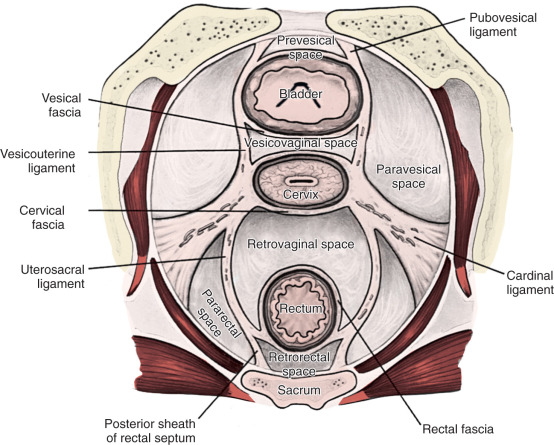
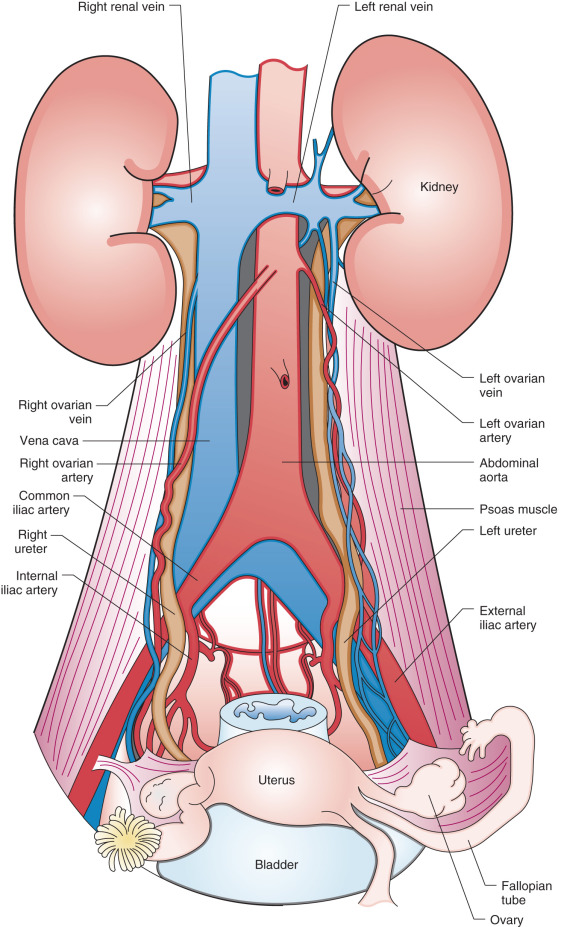
Radical hysterectomy is a procedure that must be performed by a skilled technician with sufficient experience to make the morbidity acceptable (1%–5%). The procedure involves removal of the uterus, the upper 25% of the vagina, the entire uterosacral and uterovesical ligaments ( Fig. 3.18 ), and all of the parametrium on each side, along with pelvic node dissection encompassing the four major pelvic lymph node chains: ureteral, obturator, hypogastric, and iliac. Metastatic lesions to the ovaries are rare, and preservation of these structures is acceptable, especially in young women with small lesions. The procedure is complex because the tissues removed are in close proximity to many vital structures such as the bowel, bladder, ureters ( Figs. 3.19 and 3.20 ), and great vessels of the pelvis. The object of the dissection is to preserve the bladder, rectum, and ureters without injury but to remove as much of the remaining tissue of the pelvis as is feasible.
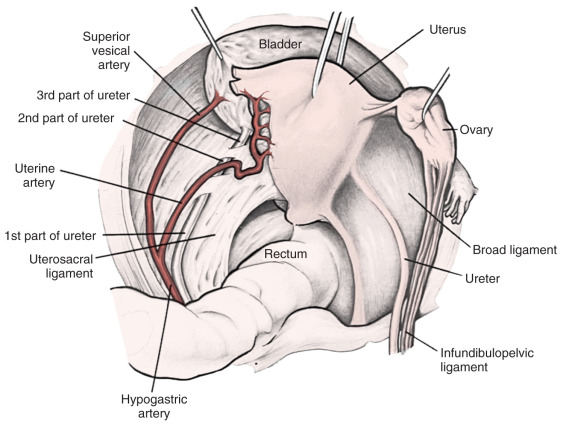
There is no doubt that in stage I and in the more restricted stage II cases, surgical removal of the disease is feasible. The addition of pelvic lymphadenectomy to the operative procedure caused considerable controversy in the early part of the century. Wertheim removed nodes only if they were enlarged and then not systematically. He believed that when accessible regional nodes were involved, the inaccessible distant nodes were also involved, and removal of suspicious nodes was more for prognostic than therapeutic value. He thought that node involvement was a measure of the lethal quality of the tumor and not merely a mechanical extension of the disease. The operative procedure popularized by Meigs included meticulous pelvic lymphadenectomy. Meigs demonstrated a 42% 5-year survival rate in another series of patients with positive nodes. Lymphadenectomy is now an established part of the operative procedure for any patient with disease greater than stage Ia1. There has been some interest in combining a radical vaginal operation with a retroperitoneal lymphadenectomy, and the results reported by Mitra, Navratil and Kastner, and McCall are surprisingly good. The survival rate in patients with negative nodes is usually in the range of 90% or more.
Rutledge and colleagues devised a system of rating radicality of hysterectomy ( Table 3.9 ) used in treating women with cervical cancer at the MD Anderson Hospital. He suggested that the term “radical hysterectomy” is not adequate to record and communicate the different amounts of therapy attempted and the subsequent risk of complications when different surgeons report their results. These authors believed that describing the technical features of five operations enabled them to evaluate more accurately their results and provided a better understanding of the need to tailor each patient’s treatment by using an operation that was adequate but not excessive.
| Class | Description | Indication |
|---|---|---|
| I | Extrafascial hysterectomy; pubocervical ligament is incised, allowing lateral deflection of the ureter | CIN, early stromal invasion |
| II | Removal of the medial half of the cardinal and uterosacral ligaments; upper third of the vagina removed | Microcarcinoma postirradiation |
| III | Removal of the entire cardinal and uterosacral ligaments; upper third of the vagina removed | Stages Ib and IIa lesions |
| IV | Removal of all periureteral tissue, superior vesical artery, and three fourths of the vagina when preservation of the bladder is still possible | Anteriorly occurring central recurrences |
| V | Removal of portions of the distal ureter and bladder | Central recurrent cancer involving portions of the distal ureter or bladder |
The goal of the class I hysterectomy was to ensure removal of all cervical tissue. Reflection and retraction of the ureters laterally without actual dissection from the ureteral bed allows one to clamp the adjacent paracervical tissue without cutting into the side of the cervical tissue itself. Class I operations are advocated primarily for in situ and true microinvasive carcinomas of the cervix. A class I procedure is also performed after preoperative radiation in adenocarcinoma of the cervix or after preoperative radiation in the so-called barrel-shaped endocervical SCC. The operation described is essentially the extrafascial hysterectomy used routinely at the MD Anderson Hospital.
Class II extended hysterectomy is described as a modified radical hysterectomy. The purpose of the class II hysterectomy is to remove more paracervical tissue ( Fig. 3.21 ) while still preserving most of the blood supply to the distal ureters and bladder. The ureters are freed from their paracervical position but are not dissected out of the pubovesical ligament. The uterine artery is ligated just medial to the ureter as it lies in “the tunnel,” ensuring preservation of the distal ureteral supply. The uterosacral ligaments are transected midway between the uterus and their sacral attachments ( Fig. 3.22 ). The medial halves of both cardinal ligaments are removed, as is the upper 25% of the vagina. A pelvic lymphadenectomy is usually performed with a class II hysterectomy. A class II operation is reported to be suitable for the following conditions:
- 1.
Microinvasive carcinomas in which the depth of invasion is considered greater than early stromal invasion
- 2.
Small postirradiation recurrences limited to the cervix.
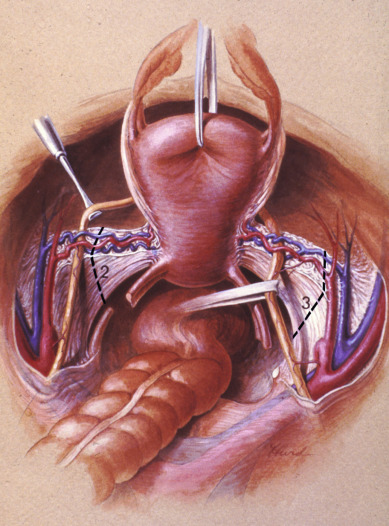
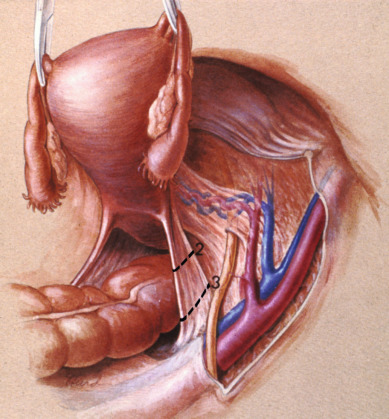
The class III procedure is a wide radical excision of the parametrial and paravaginal tissues in addition to the removal of the pelvic lymphatic tissue. The uterine artery is ligated at its origin on the internal iliac artery. In the dissection of the ureter from the pubovesical ligament (between the lower end of the ureter and the superior vesical artery), care is taken to preserve the ligament, maintaining some additional blood supply to the distal ureter. The hazard of fistula formation is decreased by preservation of the superior vesical artery along with a portion of the associated pubovesical ligament. The uterosacral ligaments are resected at the pelvic sidewall. The upper 25% of the vagina is removed ( Fig. 3.23 ), and a pelvic lymphadenectomy is routinely performed. This operation is primarily for patients with stage I or IIa carcinoma of the cervix with or without preservation of ovarian function.
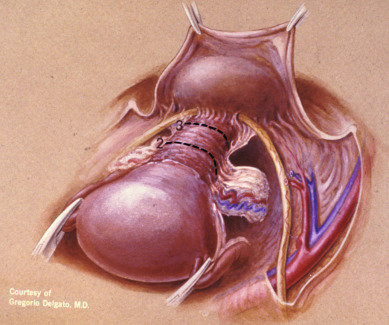
The aim of the class IV radical hysterectomy is complete removal of all periureteral tissue; a more extensive excision of the paravaginal tissues; and, when indicated, excision of the internal iliac vessels along an involved portion of the medial pelvic wall tissue. This differs from the class III operation in three respects:
- 1.
The ureter is completely dissected from the pubovesical ligament
- 2.
The superior vesical artery is sacrificed
- 3.
50% of the vagina is removed.
This procedure is used primarily for more extensive anteriorly occurring central recurrences when preservation of the bladder is seemingly still possible. Extension of the dissection laterally is needed when the disease has focally involved the medial parametrium. Sacrificing blood vessels to the bladder is unfavorable because the risk of fistula formation increases significantly. In most cases, these patients are more appropriately treated with an anterior exenteration.
The purpose of the class V hysterectomy is to remove a central recurrent cancer involving portions of the distal ureter or bladder. It differs from a class IV operation because the disease involves a portion of the distal ureter, bladder, or both, which is removed with the disease. A reimplantation of the ureter into the bladder, often as a ureteroneocystostomy, is then performed. This procedure has a rare application to a small, specifically located recurrence when exenteration is considered unnecessary or has been refused by the patient.
The modified Rutledge classification of extended hysterectomies has considerable practical value. It again underlines the necessity for the surgeon to tailor the operative procedure to the disease extent. A stage Ia2 lesion does not need an operative procedure that is as radical as the procedure for a large IIa lesion. This is particularly pertinent in the decision between a class II and class III radical hysterectomy. In many countries, the class II radical hysterectomy (called a modified radical hysterectomy) is combined with a bilateral pelvic lymphadenectomy as standard therapy for early-stage cervical cancer. Indeed, the class III type of radical hysterectomy is a phenomenon of particular prevalence in the Western hemisphere and Asia because of the dual influences of Meigs and Okabayashi. The class III, or Meigs–Okabayashi procedure, is a derivative of the Halstedian principle that a lesion should be removed en bloc with its draining lymphatics; thus, the class III radical hysterectomy calls for removal of all the parametria at the pelvic sidewall and transection of the uterosacral ligaments at the sacrum. Advocates of the modified radical hysterectomy or class II procedure with pelvic lymphadenectomy for stages I and IIa lesions suggest that the intervening lymphatics are not at risk in an early cancer of the cervix. Indeed, spread from the primary lesion to the draining pelvic wall nodes probably occurs as an embolic phenomenon. One virtually never finds a tumor in lymphatics except surrounding the primary lesions. However, it is prudent for the pathologist to take several sections of the most distal portion of the parametria following a class III radical hysterectomy for stage I or IIa cervical cancer in an effort to determine the presence or absence of malignant cells in lymphatics distant from the primary lesion. In the presence of a bulky central lesion, the need for an adequate surgical margin of resection often mandates a more extensive procedure than the typical class II radical hysterectomy. However, preservation of any portion of the lateral parametria appears to be associated with a greatly diminished incidence of bladder atony. Forney reported on 22 women extensively studied after undergoing radical hysterectomy; in 11 women, the cardinal ligaments had been divided completely, and in the other 11, the inferior 1 to 2 cm of these ligaments had been spared. Satisfactory voiding occurred significantly earlier (20 vs. 51 days) in women who had undergone an incomplete transection. In a similar manner, preservation of a portion of the uterosacral ligaments appears to be associated with fewer complaints of postoperative obstipation. Undoubtedly, the preserved tissue contains intact nerve tracts, which avoid the extensive denervation associated with the typical class III type or radical hysterectomy.
Complications
Acute complications of radical hysterectomy include pelvic hemorrhage, urinary tract injury, injury to the genitofemoral or obturator nerves, deep venous thrombosis (DVT), and pulmonary embolism. Although hemorrhage requiring transfusion of blood products is a risk of any radical hysterectomy, this complication may occur more frequently when this procedure is performed in obese patients. Soisson and colleagues reported on 43 women undergoing radical hysterectomy for early-stage cervical cancer. All patients had a body weight at least 25% greater than their ideal weight. Survival was not compromised, and the incidence of serious complications was not increased in obese patients compared with a control group. The operative technique is more difficult; the procedure lasts longer, and surgery is associated with greater blood loss.
Pulmonary embolism is the one complication most likely to cause death in the period surrounding the operative therapy of cervical cancer. This must be kept in mind at all times, and particular care must be exercised during and after surgery to avoid this devastating complication. The operative period is the most dangerous period for the formation of a thrombus in the leg or pelvic veins. Care should be taken to ensure that a constriction of veins in the leg does not occur during the operative procedure, and careful dissection of the pelvic veins should lead to minimal thrombus formation in those structures. Because of the risk of pulmonary embolism and DVT, prophylactic heparin or pneumatic compression boots (or both) are strongly recommended.
Chronic complications after radical hysterectomy include urinary dysfunction, lymphocyst formation, lymphedema, extensive abdominal scarring, fistula formation (vesicovaginal and rectovaginal), compromised sexual function, and loss of fertility. All of these complications are preventable, and the incidence is decreasing steadily ( Table 3.10 ). With highly successful surgical treatment programs in place for early-stage disease, quality of life (QoL) among survivors becomes important.
| Complication | Patients (%) |
|---|---|
| Vesicovaginal fistula | 1 |
| Ureterovaginal fistula | 2 |
| Severe bladder atony | 4 |
| Bowel obstruction (requiring surgery) | 1 |
| Lymphocyst (requiring drainage) | 3 |
| Thrombophlebitis | 2 |
| Pulmonary embolus | 1 |
The major complication after radical surgery for invasive cancer of the cervix is postoperative bladder dysfunction. Reports in the literature by Seski and Carenza and Nobili and Giacobini suggest that bladder dysfunction is a direct result of injury to the sensory and motor nerve supply to the detrusor muscle of the bladder. The more radical the surgery, the greater will be the extent of damage and the more likely postoperative bladder dysfunction will result.
This dysfunction is usually manifested in the patient by a loss of the sense of urgency to void and an inability to empty the bladder completely without the Credé maneuver. Although most patients learn to compensate for the sensory and motor loss and return to near-normal function, patients occasionally need to be taught intermittent self-catheterization, or long periods of constant bladder drainage may be necessary postoperatively. Sophisticated urodynamic studies have shown that a residual hypertonicity in the bladder detrusor muscle and urethral sphincter mechanism sometimes produces dysuria and stress incontinence. Treatment is symptomatic, with near-total recovery in most patients. Limitation of the extent and radicality of surgery, especially in patients with early lesions, can minimize this morbidity. Bandy and colleagues reported on the long-term effects on bladder function after radical hysterectomy (class III) with and without postoperative radiation. In his study, the necessity for bladder drainage of 30 or more days after surgery in 30% of patients was associated with significantly worse long-term residual and other bladder dysfunction. Adjunctive pelvic radiation was associated with significantly more contracted and unstable bladder. In a study reported from Greece of stage Ib cancers, 68 had a Rutledge type III, and 50 had a type II radical hysterectomy. Age, grade, bulky tumor, and lymph node metastasis were similar in the two groups. Postoperative radiation was given to 31% of type III and 64% of type II hysterectomies. Major complications, mainly voiding problems, were significantly more common in those treated with type III hysterectomy; however, the disease-free survival was better in the class III hysterectomy (86.5% vs. 76.5%, P <0.05). This study would suggest type III surgery is better than type II plus radiation.
Ureteral fistulas are now infrequent (0%–3%), primarily as a result of the improvement in techniques, such as avoiding excessive damage to the structure itself and preserving alternate routes of blood supply. With respect to lymphocyst formation, two studies testing the hypothesis that avoiding reperitonealization of the pelvic peritoneum obviates the need for such drainage have been reported; both studies suggest that drainage is not necessary if the peritoneum is left open over the surgical site. Ligation of the lymphatics entering the obturator fossa under the external iliac vein helps reduce the flow of lymph into this area, where lymphocyst formation is prevalent. Lymphocysts, if present, rarely cause injury and are usually reabsorbed if given enough time. Choo and colleagues reported that cysts smaller than 4 to 5 cm usually resolve within 2 months and that only observation is necessary. Surgical intervention is necessary when there is some evidence of a significant ureteral obstruction. During laparotomy, the surgeon should unroof the lymphocyst and prevent re-formation by suturing a tongue of omentum into the cavity (internal marsupialization). Percutaneous aspiration of the cyst, which is often associated with subsequent infection, should be used cautiously.
Preservation of ovarian function is often desirable for patients who must undergo a surgical procedure for invasive cancer of the cervix. Often, after a careful histologic examination of the operative specimen, including the pelvic lymph nodes, a postoperative recommendation for pelvic radiation is indicated. Standard pelvic placement of preserved ovaries will result in postirradiation ovarian failure; therefore, a procedure for transposition of the ovaries to an extrapelvic site ( Fig. 3.24 ) has been devised. Shielding during postoperative pelvic irradiation is possible with the ovaries so placed. The ovaries receive some radiation but not usually enough to prevent continued steroid production. A word of caution has been interjected by Mann and others regarding the rare occurrence of occult metastases to the ovary in patients with adenocarcinoma of the cervix. The two largest studies suggest that the incidence is between 0.6% and 1.3%. Most patients with metastatic disease in the ovary are postmenopausal or have had gross adnexal pathology or positive pelvic lymph nodes. These guidelines can be helpful in identifying patients for whom preservation of ovarian tissue is unwise. The incidence of occult metastasis to the ovary from SCC of the cervix (stages I and IIa) is so rare that preservation of ovarian tissue does not carry the same concerns. Lateral ovarian transposition will be discussed further in the following section on fertility preservation.
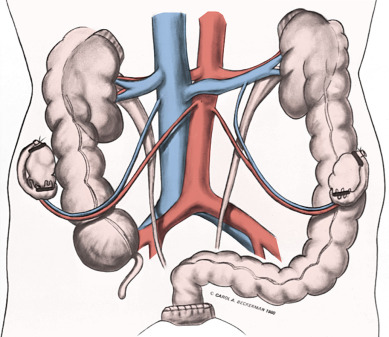
Indications for Postoperative Adjuvant Therapy
Table 3.11 contains data sets from two randomized studies that have established the role for adjuvant therapy after radical surgery based on intermediate and high-risk surgicopathologic factors. In a GOG study (protocol 92) of 277 patients with intermediate risk factors, stage Ib cancers were randomized to radical hysterectomy with or without postoperative irradiation. Of these patients, 137 were randomly assigned to radiation therapy, and 140 were not given further treatment. Based on a previous GOG study, intermediate risk was defined as greater than one-third of stromal invasion, lymph space involvement, and large clinical tumor diameter. (Note that the term lymph space involvement is used interchangeably with vascular space involvement .) All patients had negative lymph nodes. Four combinations of the risk factors were developed. Tumor size varied to greater than 4 cm. Although the two arms were equal with regard to the four combinations, in which group(s) the recurrences occurred is not stated. Using a one-tail test, there is a greater recurrence-free survival for the combined group (84.6% compared with 72.1%). OS was not significant; 11% died of cancer compared with 18% of the radical hysterectomy-only group. There was a 10% noncompliance rate in the radiation group. These authors are able to apply this information to clinical practice because more than 90% of patients would be treated without benefit with regard to survival. Data were presented with an intent-to-treat evaluation (see Table 3.11 ).
| Randomized Trial of Adjuvant Pelvic Radiotherapy for FIGO Stage IB | |||
|---|---|---|---|
| GOG 92 * | n | Recurrence (%) | RR † |
| Adjuvant pelvic RT | 137 | n = 21 (15) | 0.53, P = 0.008 |
| No further therapy | 140 | n = 39 (28) | |
| Randomized Trial of Synchronous Adjuvant Radiation Therapy and Chemotherapy for FIGO Stage IA2–IIA | |||
| GOG 109/SWOG 8797/RTOG 91-12 ‡ | n | Projected PFS (%) | Projected OS (%) |
| Adjuvant pelvic RT + CT | 127 | 80 HR 2.01, P = 0.003 | 81 HR 1.96, P = 0.007 |
| Adjuvant pelvic RT alone | 116 | 63 | 71 |
* All patients underwent radical hysterectomy and lymphadenectomy, followed by randomization based on intermediate surgicopathologic risk factors (ie, depth of stromal invasion, tumor size, presence of angiolymphatic space involvement).
† Reduction in the relative risk of recurrence.
‡ All patients underwent radical hysterectomy and lymphadenectomy followed by randomization based on high surgicopathologic risk factors (ie, metastatic tumor to the lymph nodes, parametria, or vaginal margin).
Patients with positive pelvic nodes usually receive postoperative whole-pelvis irradiation and chemotherapy (see Table 3.11 ). Peters and colleagues randomized 268 patients with FIGO stage Ia2, Ib, and IIa carcinoma of the cervix, initially treated with radical hysterectomy and pelvic lymphadenectomy and found to have positive pelvic lymph nodes or positive margins or microscopic involvement of the parametrium, to receive either pelvic radiation therapy alone or concurrent chemoirradiation. Among the 243 patients who were accessible, progression-free survival (PFS) and OS were significantly improved in the patients receiving chemotherapy. The projected PFS at 4 years was 63% with pelvic radiation therapy alone and 80% with concurrent chemoirradiation (HR, 2.01; P = 0.003). The projected OS rate at 4 years was 71% with pelvic radiation therapy alone and 81% with concurrent chemoirradiation (HR, 1.96; P = 0.007). The combined therapy arm had more frequent grade III and IV4 hematologic and gastrointestinal (GI) toxicity. This landmark intergroup study of the GOG (protocol 109), the Southwest Oncology Group (protocol 8797), and the Radiation Therapy Oncology Group (protocol 91–12) was one of five randomized trials to be published between 1999 and 2000 attesting to the value of radiosensitizing chemotherapy in the management of cervical cancer (discussed further later).
Prognostic factors have been evaluated by several authors in patients with early-stage disease who have been treated surgically. In a study by Francke and associates, 105 patients with stage Ib were treated with radical hysterectomy and had negative lymph nodes. Only LSI showed significant correlation with local failure. There were 32 patients with SCC and positive LSI, 17 received postoperative radiation with 0 of 17 recurrences, and 4 of 15 (27%) treated with surgery only developed recurrence. The OS rate at 5 years was 96% in those treated with radiation and 93.3% in those with LSI not treated with radiation. Stockler and colleagues evaluated 194 patients with stage Ib and IIa who were treated with radical hysterectomy and had negative nodes. Nuclear grade 2 or 3 ( P = 0.02) and small cell squamous histology ( P = 0.001) were each associated with a fourfold increase in risk of recurrence, but LSI ( P = 0.02), age younger than 36 years ( P = 0.03), and either tumor size greater than 28 mm ( P = 0.03) or surgical clearance less than 5 mm ( P = 0.02) was associated with a 2.5-fold increase in risk of recurrence. Survival data were not given. Delgado, in reporting a GOG study of 645 women with stage Ib squamous carcinoma, including 100 patients with positive nodes, found depth of invasion, tumor size, and lymph space involvement to be important risk factors for recurrence in multivariate analysis. The group from Boston evaluated 171 patients with lymph node–negative stage Ib and IIa cervical cancer treated primarily with surgery. A total of 116 (68%) patients were treated with surgery only, and 55 (32%) received radiation. Overall, 28 patients (16%) developed recurrent disease with no difference in the two treatment groups. After correction for other factors, patients with LSI who received radiation were less likely to develop recurrence than similar patients treated with surgery only ( P = 0.04); however, OS was similar in the two groups. In a study from Gateshead, United Kingdom, 527 patients with stage Ib to IIb cervical cancer treated with radical hysterectomy were evaluated. There were 102 (19.3%) with lymph node metastasis. In those with lymph node metastasis, histologic differentiation ( P = 0.009), and metastatic extent ( P = 0.045) were the only independent prognostic factors for risk of cervical cancer deaths.
Shibata and colleagues evaluated adjuvant chemoradiation in 37 patients who had undergone radical hysterectomy with pelvic lymphadenectomy. In addition to accepted high-risk surgicopathologic criteria (eg, positive pelvic lymph nodes or positive surgical margins), the investigators also included the presence of lymphovascular space involvement (LVSI) as part of their inclusion criteria for protocol eligibility. Adjuvant chemotherapy consisted of cisplatin (70 mg/m 2 on day 1) and 5-fluoracil (5-FU) (700 mg/m 2 /per day on days 1–4) every 4 weeks for a total of three cycles. Pelvic radiotherapy was started concurrently with the first cycle of chemotherapy and administered to a dose of 45 Gy in 25 fractions. The incidence of grade III/IV toxicities included neutropenia (24.3%), nausea and vomiting (8.1%), and diarrhea (18.9%). The 5-year PFS was 89.2%. Both the GOG and the Korean GOG have emphasized the importance of further improvement of outcomes of women found to have high-intermediate surgicopathologic risk factors (eg, large tumor diameter, deep stromal invasion, LVSI) after radical surgery for early-stage disease. A prospective, randomized trial involving adjuvant pelvic radiotherapy versus adjuvant single agent weekly cisplatin-based chemoradiation has recently been designed through collaboration of both cooperative groups.
The optimal adjuvant therapy for patients found to have high-risk surgicopathologic factors has not yet been determined. Specifically, the role of adjuvant chemotherapy in place of or in addition to adjuvant chemoradiation has been the subject of much debate. Takeshima and colleagues treated 65 consecutive patients with FIGO stage IB to IIA cervical carcinoma with adjuvant chemotherapy after radical hysterectomy and pelvic lymphadenectomy. Patients with intermediate-risk factors (stromal invasion >50%; n = 30) and those with high-risk factors (positive surgical margin, parametrial invasion, or lymph node involvement; n = 35) were treated with bleomycin, vincristine, mitomycin, and cisplatin (three cycles for intermediate-risk cases and five cycles for high-risk cases). Chemotherapy was well tolerated with no significant adverse effects and no cases of severe bleomycin-related pulmonary toxicity. The 5-year disease-free survival was 93.3% for the intermediate-risk group and 85.7% for the high-risk group. All patients with SCCs in the intermediate-risk group and 89.3% of high-risk patients with SCC remained disease free. Despite the absence of adjuvant pelvic radiotherapy, local-regional recurrence occurred in 3.3% of the intermediate-risk group and in 8.6% of the high-risk group. A randomized trial comparing adjuvant chemoradiation with and without continued systemic “consolidation” platinum-based and taxane-based chemotherapy is being considered by the GOG for patients found to have high-risk surgicopathologic factors after radical surgery.
To summarize, the recommendation for postoperative adjuvant therapy (pelvic radiotherapy with or without radiosensitizing chemotherapy) after radical hysterectomy is based on high-intermediate risk surgicopathologic factors (ie, tumor diameter, depth of stromal invasion, and presence of LVSI) and high-risk surgicopathologic factors (ie, presence of tumor at the vaginal margin, in the parametria, or in the lymph nodes). The major research questions center around limiting the toxicity of chemoradiation, continued intravenous systemic therapy, and the triage of patients with locally advanced resectable lesions (ie, IB2-IIA) to primary chemoradiation versus potentially trimodality therapy (ie, radical surgery followed by adjuvant chemoradiation).
Sexual Function
The subject of sexual function after therapy for cervical cancer is often ignored. Many patients never regain pretreatment sexual function. Patients treated with full pelvic irradiation therapy (ie, external-beam and vaginal brachytherapy) experience decreased sexual function resulting from vaginal stricture formation with obliteration and premature ovarian failure (see forthcoming discussion). Andersen studied the sexual behavior, the level of sexual responsiveness, and the presence of sexual dysfunction of 41 women with uterine cancer compared with a matched group of healthy women. The two groups were similar until the onset of signs of disease, which sometimes occurred long before diagnosis, at which time the patients with cancer began experiencing significant sexual dysfunction. Sexual morbidity therefore begins actually in the prediagnosis period for many patients. Seibel reported on 46 patients who were interviewed more than 1 year after treatment for carcinoma of the cervix to establish the effects of radiation therapy and of surgical therapy on sexual feelings and performance. The patients who were irradiated experienced statistically significant decreases in sexual enjoyment, opportunity, and sexual dreams. The surgically treated group had no significant change in sexual function after treatment. Both groups experienced a change in self-image but did not believe that their partners or families viewed them differently. Myths about cancer and the actual effects of pelvic irradiation were found to have disrupted the sexual marital relationships of many women. Therapeutic programs with counseling and vaginal rehabilitation with the use of estrogen vaginal creams and possibly the use of dilators may be beneficial. Although radical hysterectomy offers an enhanced functional outcome, the procedure does not always leave sexual function undisturbed, with orgasmic problems and dyspareunia resulting from reduced vaginal size having been reported.
Nerve-Sparing Radical Hysterectomy
Lin and colleagues evaluated urodynamic function in 20 women with cervical cancer who underwent radical hysterectomy. Urodynamic parameters measured preoperatively and postoperatively included bladder voiding and bladder storage functions, both of which were found to be significantly impaired in all 20 cases after surgery. Surgical damage to the pelvic autonomic nerves is likely to be responsible for not only subsequent impaired bladder function but also in defecation problems and sexual dysfunction. The development of a nerve-sparing procedure that does not compromise the radicality of the operation is highly desirable.
Trimbos and colleagues introduced elements of the Japanese nerve-sparing technique in their Dutch population, citing that in various Japanese oncology centers, it had been recognized that the anatomy of the pelvic autonomic nerve plexus permits a systematic surgical approach to preserve these structures. The investigators first identified and preserved the hypogastric nerve in a loose tissue sheath underneath the ureter and lateral to the uterosacral ligament; next, the inferior hypogastric plexus in the parametrium is lateralized and avoided during parametrial transection; finally, the most distal part of the inferior hypogastric plexus is preserved during the dissection of the posterior part of the vesicouterine ligament. Trimbos and colleagues concluded that the procedure is feasible and safe and deserves further consideration.
An updated series was presented by Maas and colleagues, who observed that the incidence of urinary dysfunction appears to be very low after nerve sparing. These findings have been supported by an Italian series of 23 patients reported by Raspagliesi and colleagues and by two recent Japanese papers for which urodynamic data were recorded for 27 patients. In the study by Sakuragi and colleagues, none of 22 patients for whom the nerve-sparing procedure was performed had urinary dysfunction, compared with three of the five patients for whom the procedure could not be performed.
Nerve-sparing radical hysterectomy is an attractive approach because of improved urogenital, anorectal, and sexual functions. The sympathetic fibers that innervate the uterus, vagina, urinary bladder, and rectum come from T11 to L2 and form the superior hypogastric plexus. The parasympathetic fibers come from S2 to S4 at the pelvic wall as the pelvic splanchnic nerve. These fibers merge and form the inferior hypogastric plexus and branch to innervate the uterus and the urinary bladder. Professor Fujii from the Kyoto University Gynecology Group has gone to great lengths to provide a step-by-step anatomic identification of the nerve-sparing radical hysterectomy ( Table 3.12 ). The first step in the nerve-sparing procedure involves isolation and separation of the deep uterine vein from the pelvic splanchnic nerve ( Fig. 3.25 ). This is then followed by isolation and separation of the hypogastric nerve. Ultimately, the bladder branch from the inferior hypogastric plexus is identified running parallel to the blood vessels in the paracolpium, and the uterine branch from the inferior hypogastric plexus is separated and divided ( Fig. 3.26 ). This procedure can be accomplished only through meticulous division of the posterior leaf of the vesicouterine ligament. Through separation of the inferior vesical vein in this ligament, the bladder branch from the inferior hypogastric plexus can be identified and preserved.
| Nerve-Sparing Radical Hysterectomy | Traditional Radical Hysterectomy | P Value | |
|---|---|---|---|
| FIGO stage IB1 | 10 | 8 | ns |
| FIGO stage IB2 | 0 | 1 | |
| FIGO stage IIA | 0 | 1 | |
| Mean operative time (min) | 197 | 155.5 | 0.05 |
| Median lymph nodes removed ( n ) | 17.8 | 15.7 | NS |
| Hospital stay (days) | 7.6 | 8.4 | NS |
| Postvoid residual urine volume >50 mL (days) | 3.5 | 9.1 | 0.00078 |
| Mean decrease in hgb concentration (g%) | 1.7 | 2.5 | ns |
| Complications | 1 (blood transfusion) | 0 | ns |
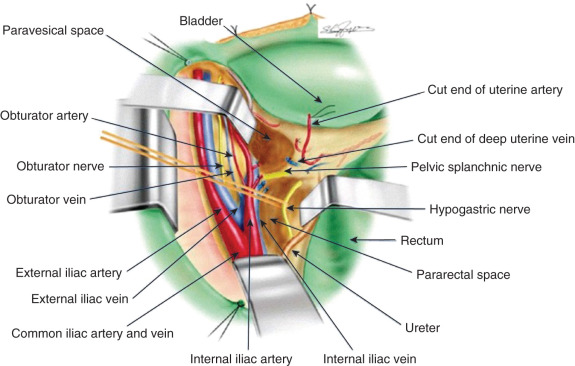
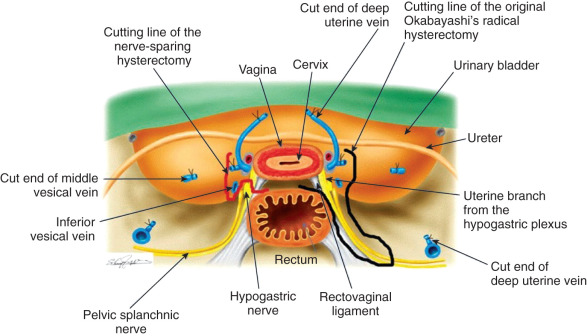
van den Tillaart and colleagues evaluated the feasibility, safety, and local recurrence rate in 122 patients with FIGO stage IA to IIA lesions and compared them with 124 patients who underwent non–nerve-sparing surgery. Unilateral or bilateral sparing of nerves was possible in 80% of cases in the nerve-sparing group. Operative time and blood loss were less in the nerve-sparing group, but the postoperative courses were similar. The local recurrence rates were not significantly different at 2 years of follow-up. Nerve-sparing surgery was not a significant prognostic factor for local recurrence in univariate and multivariate regression analyses. Neuronavigation systems and robotic-assisted nerve-sparing techniques continue to be under investigation along with the incorporation of the nerve-sparing procedure to radical trachelectomy for fertility preservation.
From 2003 to 2005, Roh et al. randomly assigned 92 women with FIGO stage IA2 to IIA disease to conventional radical hysterectomy or nerve-sparing surgery. The patients were well-balanced in clinicopathologic characteristics, and among 86 patients included in final analysis, the frequency of long-term urinary symptoms was higher in the conventional surgery group, with significant deterioration of volume of residual urine and bladder compliance at 12 months. There were no significant differences observed in the 10-year disease-free survival between groups.
In 2009, Querleu and Morrow proposed a new classification system for radical hysterectomy that continues to gain acceptance, particularly in Europe. In this new scheme, four main categories are described according to the extent of removal of the paracervix, using only the ureter, internal iliac vessels, and pelvic wall as reliable landmarks. The new system also incorporates nerve-preservation techniques and paracervical lymphadenectomy in subcategory assignments. In class A, the cervix is removed completely with minimal removal of the paracervix; in class B, the paracervix is removed to the level of the ureter. In class C, the entire paracervix is removed at the pelvic sidewall, and class D describes various exenterative procedures. Class C1 allows for preservation of the autonomic nerves, class D1 includes removal of the vascular parietal system, and class D2 requires removal of muscle and fascia at the lateral pelvic wall.
Cibula et al. have approached the new classification scheme by Querleu and Morrow by developing it further into a three-dimensional (3D) model using the standard anatomic landmarks for definition of resection margins in longitudinal and transverse dimensions. In their 2011 manuscript, figures included photographs of the resulting 3D anatomic template that presents the parametrial resection in three dimensions along with the nerve-sparing procedure. The investigators intent is to increase adoption of the new classification scheme within the oncologic community and to enhance harmonization of clinical practice as it pertains to radical hysterectomy.
Sentinel Lymph Node Identification
Although the risk of nodal metastases is low in women with small, early cancers (ie, FIGO Ia2 and Ib1 lesions), the need for bilateral pelvic lymphadenectomies must still be emphasized. Controversy has centered on the existence and ability to identify sentinel lymph nodes (SLNs) in cervical cancer. The rationale for identifying an SLN in cervical cancer is to avoid full pelvic lymphadenectomies, which can result in lymphocyst formation and lower-extremity lymphedema, especially when adjuvant pelvic radiotherapy is given. Two techniques for sentinel node identification are available. An injection is performed around the tumor using either a blue dye or an isotopic colloid. Ideally, the two techniques are used concomitantly in patients with early-stage lesions ( Fig. 3.27 ).
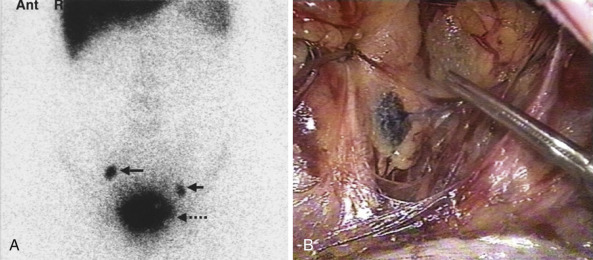
Dargent and Enria reported the results on 70 consecutive patients. Failure in identification of the SLN occurred in 14 of the 139 attempted dissections. One SLN was detected in 121 dissections, and two SLNs were detected in four dissections. The investigators carried out a systematic pelvic lymphadenectomy after removal of the SN. A metastatic involvement of the SLN was put in evidence in 19 of the 129 retrieved SLNs. The other regional lymph nodes were involved in 13 cases and not involved in six cases. In the 110 cases in which the SLN was not involved, all of the other regional nodes were free from metastasis.
Table 3.13 contains studies on SLN detection in cervical cancer from 2007 to 2010. Most studies were performed with a combination of blue dye and a radioactive tracer with detection rates ranging from 87.3% to 100%. False-negative rates have ranged from 0% to 22.6%. Darlin and colleagues noted that the technique appears to be an accurate method for identifying lymph node metastases in cervical cancer patients with tumors that are 2 cm or smaller. The investigators recommend that in the case of a unilateral SLN only, a complete lymphadenectomy should be performed on the radionegative side. Although a randomized clinical trial to assess the equivalency of SLN mapping will not be feasible because a sample size of more than 1800 patients will be required to evaluate a decrease in recurrence rate from 15% to 10%, the GOG is prospectively studying the utility of lymphatic mapping and SLN identification in patients with early-stage cervical cancer (GOG protocol 206).
| Reference | Patients ( n ) | Technique | Detection Rate (%) | Metastases Sentinel Node (%) | False-Negative (%) |
|---|---|---|---|---|---|
| Frumovitz et al. | 831 | R/B | 89.8 | 20.5 | 8.2 |
| Bats et al. | 71 | R/B | 87.3 | 25.8 | 11 |
| Altgassen et al. | 590 | R/B | 88.6 | 15.7 | 22.6 |
| Diaz-Feijoo et al. | 50 | R/B | 100 | Unknown | 0 |
| Kara et al. | 32 | R/B | 100 | 28.1 | 0 |
| Fader et al. | 38 | R/B | 92.1 | 15.7 | 16.7 |
| Van de Lande et al. | 58 | R/B | 96.6 | 21.4 | 0 |
| Pluta et al. | 60 | R/B | 100 | 8.3 | 0 |
Laparoscopic Radical Hysterectomy With Lymphadenectomy
Advantages of minimally invasive surgery include magnification for improved visualization, decreased blood loss, shorter hospitalization, more rapid recovery to full function, less adhesion formation, improved cosmesis, and decreased wound complications (eg, infection, hernia formation). A type III Wertheim–Meigs radical hysterectomy with bilateral pelvic and aortocaval lymphadenectomies can be accomplished via operative laparoscopy and has been reported by several centers ( Table 3.14 , Fig. 3.28 ). Malzoni and colleagues published a series of 77 patients, 60 of whom underwent a class III operation and 17 who underwent a class II operation. The median body mass index (BMI) was 27 kg/m2 (range, 19–35 kg/m 2 ). Mean operative time was 186 minutes, median blood loss was 57 mL (range, 30–90 mL), and the median hospital stay was 4 days (range, 3–7 days). Chen and colleagues performed 290 laparoscopic radical hysterectomies with lymphadenectomy. The median blood loss was 230 mL (range, 50–1200 mL), and the mean operative time was 162 minutes (range, 110–350 minutes). Positive lymph nodes were detected in 27.1%, and surgical margins were clear in all patients. Postoperative complications occurred in 10.8% and included ureterovaginal fistula ( n = 5), vesicovaginal fistula ( n = 4), ureterostenosis ( n = 3), DVT ( n = 9), lymphocyst ( n = 4), lymphedema ( n = 5), and trocar insertion site metastasis ( n = 1). Recurrent disease occurred in 16.3% ( n = 48) at a median follow-up of 36.5 months.
| Authors | Patients ( n ) | BMI | OR Time (min) | EBL (mL) | Hospital Stay (days) | Transfusion | Pelvic Lymph Nodes |
|---|---|---|---|---|---|---|---|
| Spirtos et al. | 78 | ≤35 | 205 | 225 | 2.9 | 1 | 34 |
| Ramirez et al. | 20 | 26 | 332.5 | 200 | 1 | 1 | 13 |
| Gil Moreno et al. | 27 | 26 | 285 | 400 | 5 | 2 | 19.1 |
| Abu-Rustum et al. | 19 | 23.1 | 371 | 301 | 4.5 | 1 | 25.5 |
| Pomel et al. | 50 | 21.5 | 258 | 200 | 7.5 | 1 | 13.22 |
| Nezhat et al. | 7 | — | 293 | 143 | 3.2 | — | 27.8 |
| Puntambekar | 248 | — | 92 | 165 | 3 | — | 18 |
| Frumovitz | 35 | 28.1 | 344 | 319 | 2 | 6 | 14 |
| Malzoni et al. | 77 | 27 | 186 | 57 | 4 | 0 | 23 |
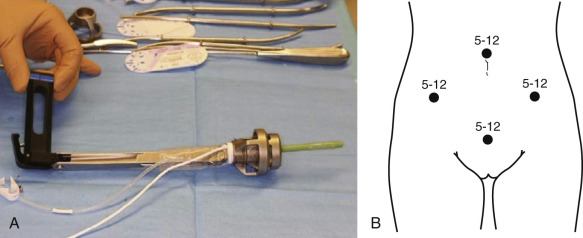
Single-site laparoscopic surgery platforms have been developed and are being evaluated in gynecologic oncology centers. Reports of this technique using the multichannel single-incision laparoscopic surgery port for laparoscopic cases or a single channel Gelport for robotic-assisted cases on the da Vinci Surgical System in the performance of radical hysterectomy with lymphadenectomy using articulating endoscopes and articulating instrumentation are forthcoming.
Robotic-Assisted Laparoscopic Radical Hysterectomy With Lymphadenectomy
Worldwide adoption of a minimally invasive surgical approach for radical hysterectomy with lymphadenectomy using standard laparoscopic techniques has been slow because of technical difficulties, long surgeon learning curve, and long operative time in many cases. The counterintuitive hand movements, two-dimensional visualization, and limited degrees of instrument motion within the body in addition to ergonomic difficulty and tremor amplification constitute additional obstacles. The development of the robotic-assisted laparoscopic technique using the da Vinci Surgical System has allowed for high-definition 3D visualization, instruments that increase surgical accuracy by mimicking the complex movements of the human hand, enhanced dexterity with tremor abolition, and faster suturing ( Fig. 3.29 ). In both laboratory drills and in clinical settings, robotic-assisted laparoscopic techniques appear to require a shorter learning curve.
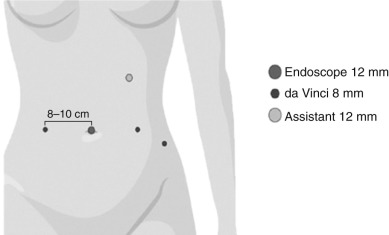
During the preceding 5 years, reports of robotic-assisted laparoscopic radical hysterectomy with lymphadenectomy has started appearing in the literature ( Table 3.15 ). Maggioni and colleagues compared the surgical outcome of robotic radical hysterectomy ( n = 40) with that of radical abdominal hysterectomy ( n = 40 historic cases). The two groups did not differ significantly in BMI, stage, histology, or intraoperative complications. The median age did differ significantly (44 years in the robotic cohort vs. 49 years in the historic group; P = 0.035). The mean operative time was significantly shorter for the laparotomy group (mean, 200 minutes vs. 272 minutes; P = 0.0001), but the mean blood loss was statistically significant in favor of the robotic group (78 mL vs. 222 mL; P <0.0001). A higher number of pelvic lymph nodes was removed via laparotomy (mean, 26 nodes vs. 20 nodes; P <0.05). The mean length of stay was significantly shorter for the robotic group. There were no significant differences in terms of postoperative complications between the two groups.
| Robotic ( n = 32) | Laparoscopic ( n = 17) | Laparotomy ( n = 14) | P Value | |
|---|---|---|---|---|
| IA2 | — | 2 (11.8%) | — | NS |
| IB1 | 29 (90.6%) | 14 (82.4%) | 13 (92.9%) | NS |
| IB2 | 3 (9.4%) | 1 (5.9%) | 1 (7.1%) | NS |
| BMI (mean) | 29.7 | 28.1 | 29.5 | NS |
| Operative time (mean) | 2.4 hr | 2.2 hr | 1.9 hr | 0.05 (robotic vs. laparotomy) |
| EBL (mean) | 130 cm 3 | 209.4 cm 3 | 621.4 cm 3 | 0.009 (robotic vs. laparoscopy); <0.0001 (robotic vs. laparotomy) |
| Total nodes (mean) | 32.4 | 18.6 | 25.7 | <0.001 (robotic vs. laparoscopy); <0.05 (robotic vs. laparotomy) |
| Positive margins | 5 (15.6%) | 3 (17.7%) | 3 (21.4%) | ns |
Finally, Cantrell and colleagues reported their 3-year experience involving 63 patients who underwent successful robotic-assisted laparoscopic type III radical hysterectomy. There was one intraoperative complication (asystole after induction) and two postoperative complications (intensive care unit admission for cardiac evaluation and reoperation for vaginal cuff dehiscence). Thirty-two percent of patients received adjuvant therapy. At a median follow-up period of 12.2 months, there has been recurrence and death of one patient, resulting in a 94% PFS and OS. These survival outcomes did not differ significantly from those of a historic cohort at the investigators’ institution.
Robotic-assisted techniques for the management of early-stage cervical carcinoma appear to be feasible, but continued evaluation of oncologic outcomes and a cost–benefit analysis is still needed. Important complications that also require careful evaluation and scrutiny are those that may be specific to robotic techniques, including adverse effects associated with prolongation of steep Trendelenburg position, herniation at 8-mm port sites, vaginal cuff dehiscence, freezing of the robotic arms intraoperatively, and consequences of collisions. In addition, there has been a report of an 8-mm robotic port-site metastasis in a patient who underwent robotic-assisted laparoscopic radical hysterectomy and bilateral pelvic lymphadenectomy for a node-negative FIGO stage IB1 adenocarcinoma of the cervix.
A systematic review and meta-analysis of robotic radical hysterectomy for early-stage disease was recently performed by Shazly et al. Using MEDLINE, EMBASE, and SCOPUS from the time of database inception through February 2015, 26 nonrandomized studies enrolling 4013 women were identified. Robotic radical hysterectomy was associated with significantly less blood loss and blood transfusion, lower febrile morbidity, shorter hospital stay, and decreased wound-related complications compared with abdominal radical hysterectomy. Compared with laparoscopic radical hysterectomy, robotic radial hysterectomy was associated with comparable intra- and postoperative outcomes.
Fertility-Preserving Surgery for Early-Stage Tumors
For patients with microinvasive cervical carcinoma, management depends on the depth of invasion, and select patients may undergo conservative treatment with either cervical conization (FIGO Ia1) or radical trachelectomy with lymphadenectomy (FIGO Ia1 with LVSI, FIGO Ia2, and FIGO Ia adenocarcinoma) (see Fig. 3.17 ). In addition, patients with FIGO stage Ib lesions smaller than 2 cm with limited endocervical involvement and no pathologic evidence of lymph node metastases may be candidates for radical trachelectomy. In patients who are selected for conservative therapy, there should be no clinical evidence of impaired fertility and a strong desire for future childbearing. In addition, close surveillance should be instituted with scheduled Pap testing, colposcopic evaluation, and endocervical curettage.
Cervical Conization for Adenocarcinoma in Situ and Microinvasive Carcinoma
There has been a movement during the past decade to seriously explore fertility-sparing surgery for patients with adenocarcinoma in situ (AIS) and microinvasive carcinomas. For many patients with AIS, local excision appears to be sufficient treatment provided ectocervical and endocervical margins are clear and patient compliance with follow-up is demonstrable. It would appear that cold knife, CO 2 laser, and large loop excision of the transformation zone (LLETZ) conization procedures produce acceptable results; however, these approaches should be reserved for highly selected patients ( Table 3.16 ). Patients with margin involvement should be considered for repeat excisional biopsy. A 2009 meta-analysis composed of 33 studies indicated a recurrence rate for AIS of 2.6% for negative margins and 19.4% for positive margins (odds ratio [OR], 2.48; 95% CI 1.05, 1.622; P <0.001). Invasive adenocarcinoma was more commonly associated with positive margins (5.2%) compared with negative margins (0.1%).
| Author | Depth ≤ 3.0 mm | Recurrences | Depth 3.1–5.0 mm | Recurrences |
|---|---|---|---|---|
| Schorge et al. | 5 | 0 | — | — |
| McHale et al. | 4 | 0 | — | — |
| Webb | 20 | Not specified | 18 | Not specified |
| Smith | 31 | 1 | 29 | 1 |
| Ceballos et al. | 1 | 0 | — | — |
| Poynor et al. | 2 | 0 | 1 | 0 |
| Bisseling et al. | 16 | 0 | 2 | 0 |
| Total | 79 | 1 | 50 | 1 |
In a study of 85 patients with FIGO stage IA1 SCC treated by electrosurgical conization and cold conization to preserve fertility, there was one recurrence (1.2%) at a median follow-up of 81 months. In a second report containing 75 patients with FIGO stage IA1 SCC, there were no recurrences among the 53 women who underwent conization followed by hysterectomy nor in the 22 who underwent conization alone. As in the case with AIS and microinvasive SCC, conservative fertility-preserving therapy may be considered for select cases of microinvasive adenocarcinoma, although this remains controversial. It would seem that for FIGO stage IA1 adenocarcinomas, conization is safe. When LVSI is present, laparoscopic pelvic lymphadenectomy seems advisable. In summary, for highly selected patients with early-stage disease seen in consultation with a gynecologic oncologist, cold knife cervical conization is a reasonable alternative to preserve fertility provided compliance with follow-up is not problematic and pathology review has excluded highly aggressive histologic subtypes (eg, neuroendocrine tumors).
Vaginal Radical Trachelectomy With Laparoscopic Lymphadenectomy
Radical trachelectomy involves removing all or most of the cervix along with the bilateral parametria and upper vagina. This procedure allows for preservation of the uterus for childbearing and can be performed vaginally, abdominally, or through a minimally invasive approach (ie, straight-stick laparoscopy or robotic-assisted laparoscopy). Of note, patients with stage Ia2 disease have a 6.3% risk of nodal metastases, so treatment must include a formal pelvic lymphadenectomy.
In 1987, Dargent designed a fertility-preserving operation for stage Ia2 and some Ib1 lesions. A variant of the classical Shauta operation of vaginal radical hysterectomy, the vaginal radical trachelectomy (VRT) is performed in conjunction with bilateral laparoscopic lymphadenectomies. The VRT is performed with division of the uterus underneath the isthmus, and at the completion of the procedure, the uterus is sutured to the vagina. Oncologically, the technique is satisfying because a wide margin around the lesion is obtained containing the parametria and the upper vagina but leaving the body of the uterus in situ.
Plante and colleagues collected more than 600 reports of the vaginal technique from the past decade’s literature, including 115 of her own. Oncologic outcomes have been satisfactory with an overall recurrence rate of 4.5% and death rate from disease of 2.5% ( Table 3.17 ). Risk factors for recurrence include lesions 2 cm or larger (29% vs. 1%) and the presence of LVSI (12% vs. 2%). Adenocarcinomas and adenosquamous carcinomas are not clearly associated with an increased risk of recurrence. Patients with neuroendocrine tumors should probably not be offered fertility-sparing surgery. Approximately 10% to 12% of patients selected for VRT are found to have more extensive endocervical disease at the time of surgery or positive nodes on frozen section, leading to abandoning the procedure in favor of adjuvant therapy or completion radical hysterectomy.
| Author | Patients ( n ) | Median Follow-Up (mo) | Recurrence Rate (%) | Death (%) |
|---|---|---|---|---|
| Marchiole | 118 | 95 | 7 (6) | 5 (4) |
| Plante | 115 | 74 | 4 (3) | 2 (2) |
| Shepherd | 112 | 45 | 3 (3) | 2 (2) |
| Hertel | 100 | 29 | 3 (3) | 2 (2) |
| Covens et al. | 93 | 30 | 7 (7.5) | 4 (4) |
| Sonoda | 36 | 21 | 1 (3) | 0 |
| Burnett et al. | 19 | 21 | 2 (10.5) | ? |
| Schlearth | 10 | 48 | 0 | 0 |
| Total | 603 | 27 (4.5) | 15 (2.5) |
Plante and colleagues also tabulated obstetric outcomes for 256 patients ( Table 3.18 ). Approximately 62% of pregnancies after VRT will reach the third trimester, of which 65% will reach term. The preterm delivery rate is in the range of 28%, but only 12% will end with significant prematurity (<32 weeks) in which most neonatal morbidity occurs. Overall, 40% of all pregnancies can be expected to culminate with the birth of a healthy newborn at term.
| Author | Pregnancies | TAB, Ectopic (%) | First-Trimester Loss (%) | Second-Trimester Loss (%) | Third-Trimester Deliveries (%) | Deliveries <32 wk (%) | Deliveries 33–36.6 wk (%) | Deliveries >37 wk (%) | Currently Pregnant |
|---|---|---|---|---|---|---|---|---|---|
| Plante | 87 | 4 (5) | 17 (20) | 3 (4) | 58 (66) | 3 (5) | 8 (14) | 47 (81) | 5 (5) |
| Mathevet | 56 | 5 (9) | 9 (16) | 8 (14) | 34 (61) | 2 (6) | 3 (9) | 29 (85) | 0 |
| Shepherd | 55 | 3 (5) | 14 (25) | 7 (13) | 28 (51) | 8 (29) | 12 (43) | 8 (29) | 3 (5) |
| Bernardini et al | 22 | 0 | 3 (14) | 1 (4) | 18 (82) | 3 (17) | 3 (17) | 12 (67) | 0 |
| Hertel | 18 | 2 (11) | 1 (5) | 0 | 12 (67) | — | — | — | 3 (16) |
| Sonoda | 11 | 0 | 3 (27) | 0 | 4 (36) | 0 | 0 | 4 (100) | 4 (36) |
| Burnett et al | 3 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 0 |
| Schlearth | 4 | 0 | 0 | 2 | 2 | 1 | 0 | 1 | 0 |
| Total | 256 | 14 (5) | 47 (18) | 22 (8.6) | 158 (62) | 18 (12) | 26 (16) | 102 (65) | 15 (6) |
Abdominal approaches to radical trachelectomy may include nerve-sparing, laparoscopic, or robotic-assisted techniques. Potential benefits of the abdominal approach for radical trachelectomy include wider parametrial resection, possible lower intraoperative complication rates, and techniques familiar to most gynecologic oncologists ( Fig. 3.30 ). Cibula and colleagues reviewed the more than 100 reported cases of abdominal radical trachelectomy (ART) in 2008 and then updated their own experience with the abdominal approach in 2009. Specific indications for the abdominal approach have included clear cell carcinoma of the upper vagina, clear cell cervical carcinoma in pediatric patients, cervical cancer in patients with distorted vaginal anatomy, cancer in the cervical stump after subtotal hysterectomy, bulky exophytic cervical cancer, extent and location of cervical cancer that requires increased radicality of parametrial resection (type III), and cervical cancer in the first half of pregnancy. In total, nine live births have been reported, of which at least two were premature deliveries.
Einstein and colleagues compared surgical and pathologic outcomes for 43 adult patients with FIGO stage IB1 lesions who underwent VRT ( n = 28) or ART ( n = 15) ( Table 3.19 ). The median measured parametrial length in the VRT group was 1.45 cm compared with 3.97 cm in the ART group ( P <0.0001). Parametrial nodes were only detected in the ART specimens ( n = 8, 57.3%). There was no difference in histologic subtypes, LVSI, or median total regional lymph nodes removed in the two groups. Median blood loss was greater but not clinically significant in the ART group, and the median operating time was less in the ART group. The two groups did not differ significantly in the overall complication rate. The investigators concluded that the abdominal approach allows for wider parametrial resection, including contiguous parametrial nodes.
| VRT ( n = 28) | ART ( n = 15) | P Value | |
|---|---|---|---|
| Median gross length (cm) | 1.45 (0.73–1.63) | 3.97 (2.7–5.36) | 0.01 |
| Median histologic length (cm) | 1.07 (0.89–1.25) | 1.51 (1.36–1.77) | ≤0.0001 |
| Patients with parametrial lymph nodes detected | 0 (0%) | 8 (57.3%) | 0.0002 |
Minimally invasive approaches (ie, laparoscopic and robot assisted) to radical trachelectomy were recently studied retrospectively by Vieira et al. One hundred patients from four institutions were identified with early-stage disease and treated via an open approach ( n = 58) or a minimally invasive approach ( n = 42). There were no differences in clinicopathologic factors between the two groups, including BMI, histology, FIGO stage, or the presence of lymphovascular space invasion. Intraoperative and postoperative complications did not differ significantly between the two groups. Although the pregnancy rate was higher in the open surgery group (51% vs. 28% P = 0.018), the authors note that median follow-up was shorter in the minimally invasive surgery group (25 vs. 66 months). As expected, minimally invasive approaches were associated with less blood loss and shorter hospital stays.
By allowing for the preservation of the body of the uterus and thereby the potential for reproductive function, the radical trachelectomy emerges as a true breakthrough in the management of young women with early-stage cervical cancer ( Fig. 3.31 ). It is currently the fertility-sparing procedure with the most available data supporting its use. Although these results are encouraging, there is lack of level I evidence (ie, randomized controlled trials [RCTs]) comparing safety and survival rates between conservative and radical methods. Therefore, these techniques should be used by fully trained operators. In our opinion, the technique can be considered in conjunction with laparoscopic transperitoneal lymphadenectomy in patients who strongly desire future fertility and harbor a stage Ia1 lesion with LVSI, an Ia2 lesion, or an Ib1 tumor less than 2 cm in diameter.
For patients with FIGO stage IB tumors larger than 2 cm, VRT is not recommended because of unacceptable recurrence rates. There are no clear consensus guidelines regarding the safety of abdominal radical trachelectomy, laparoscopic radical trachelectomy, or robotic radical trachelectomy for such patients. One option is to administer neoadjuvant chemotherapy to reduce tumor size. In a recent literature review by Pareja et al., different approaches to radical trachelectomy were analyzed, and the outcome measures appear in the table below:
| ART >2 cm (%) | ART All Sizes (%) | NACT Followed by Surgery (%) | VRT (%) | VRT >2 cm (%) | |
|---|---|---|---|---|---|
| Pregnancy | — | 16.2 | 30.7 | 24 | — |
| Recurrence | 6 | 3.8 | 7.6 | 4.2 | 17 |
Lateral Ovarian Transposition
As described previously, young patients with FIGO stage I to IIa cervical carcinoma who are considered to be at high risk for requiring adjuvant pelvic irradiation (with or without radiosensitizing chemotherapy) should have the ovaries transposed to the paracolic gutters at the time of radical abdominal hysterectomy. The infundibulopelvic ligament is mobilized, and two large metallic clips should be placed in an “X” formation across the mesosalpinges to assist in radiographic localization during radiation treatment planning. Patients with locally advanced carcinomas (ie, FIGO stage Ib2 to IVa) who will receive primary chemoirradiation can undergo lateral ovarian transposition via laparoscopy in anticipation of therapy.
The incidence of ovarian failure following transposition ranges from 28% to 50% when pelvic irradiation is used. There is a tendency to become postmenopausal if the scatter radiation dose at the transposed ovaries is greater than 300 cGy. This scatter radiation dose does not appear to depend on the distance the ovaries are placed from the linea innominata. The risk of premature ovarian failure when adjuvant radiation therapy is not required is approximately 5% in patients who have undergone lateral ovarian transposition. The risk of developing symptomatic ovarian cysts appears to be approximately 5%.
Husseinzadeh and colleagues performed lateral ovarian transposition in 22 patients with invasive cervical cancer, 15 of whom received whole pelvic external radiation therapy. Nine patients also received one or two intracavitary insertions. Ovarian function was measured by the serum gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Five patients developed postmenopausal symptoms. Ovarian function was preserved in seven patients, all of whom received an average dose of 250 cGy to the ovaries via external radiation and intracavitary insertion(s). FSH values ranged from 3.3 to 38.8 mIU mL-1 (mean, 17.7 mIU mL-1).
Treatment of Locally Advanced Disease
Radiotherapy
Over the past century, radiotherapy has emerged as a notable alternative to radical surgery, primarily because of improvements in technique. The number of radiation-resistant lesions was discovered to be small, and skilled radiologists limit radiation injury, especially with the moderate dosages used for early disease. Much evidence has been presented that proves that radiotherapy can destroy disease in lymph nodes and in the primary lesion. Over the past 2 decades, radical hysterectomy has been reserved in many institutions for patients who are relatively young, lean, and in otherwise good health. In other areas of the United States, radiotherapy or surgery is used alone when the alternative modality is not available. The relative safety of both treatment modalities and the high curability for stages I and IIa lesions give physicians and patients a true option for therapy.
Radiotherapy for cancer of the cervix was begun in 1903 in New York by Margaret Cleaves. In 1913, Abbe was able to report an 8-year cure. The Stockholm method was established in 1914, the Paris method in 1919, and the Manchester method in 1938. Radium was the first element used; it has always been the most important element in radiotherapy of this lesion. External irradiation was used to treat the lymphatic drainage areas in the pelvis lateral to the cervix and the paracervical tissues.
Successful radiation therapy depends on the following:
- 1.
Greater sensitivity of the cancer cell, compared with the cells of the normal tissue bed, to ionizing radiation
- 2.
Greater ability of normal tissue to recuperate after irradiation
- 3.
A patient in reasonably good physical condition.
The maximal effect of ionizing radiation on cancer is obtained in the presence of a good and intact circulation and adequate cellular oxygenation. Preparation of the patient for a radical course of irradiation therapy should be as careful as the preparation for radical surgery. The patient’s general condition should be as well maintained as possible with a diet high in proteins, vitamins, and calories. Excessive blood loss should be controlled, and hemoglobin should be maintained well above 10 g.
Some consideration must be given to the tolerance of normal tissues of the pelvis, which are likely to receive relatively high doses during the course of treatment of cervical malignancy. The vaginal mucosa in the area of the vault tolerates between 20,000 and 25,000 cGy. The rectovaginal septum is said to tolerate approximately 6000 cGy over 4 to 6 weeks without difficulty. The bladder mucosa can accept a maximal dose of 7000 cGy. The colon and rectum will tolerate approximately 5000 to 6000 cGy, but small bowel loops are less tolerant and are said to accept a maximal dose of between 4000 and 4200 cGy. This pertains to small bowel loops within the pelvis; the tolerance of the small bowel when the entire abdomen is irradiated is limited to 2500 cGy. One of the basic principles of radiotherapy is implied here: The normal tissue tolerance of any organ is inversely related to the volume of the organ receiving irradiation. External irradiation and intracavitary radium therapy must be used in various combinations ( Table 3.20 ). Treatment plans must be tailored to each patient and her particular lesion. The size and distribution of the cancer, not the stage, should be treated. Success in curing cancer of the cervix depends on the ability of the therapy team to evaluate the lesion (and the geometry of the pelvis) during treatment and then make indicated changes in therapy as necessary. Intracavitary radium therapy is ideally suited to the treatment of early tumors because of the accessibility of the portio of the cervix and the cervical canal. It is possible to place radium or cesium in close proximity to the lesion and thus deliver surface doses that approximate 15,000 to 20,000 cGy. In addition, normal cervical and vaginal tissue has a particularly high tolerance to irradiation. One therefore has an ideal situation for the treatment of cancer because there are accessible lesions that lie in a bed of normal tissue (cervix and vagina) that is highly radioresistant.
| Whole Pelvis | |||
|---|---|---|---|
| Stage | Radiation Dose (cGy) | Brachytherapy (mg/hr) | Surgery |
| Ial true microinvasive | — | — | Extrafascial hysterectomy |
| Ia2 | 2000 (2000 parametrial) | 8000 (two applications) | Radical hysterectomy with bilateral pelvic lymphadenectomy as an option |
| Ib | 4000 | 6000 (two applications) | |
| IIb | 4000–5000 * | 5000–6000 (two applications) | Consider pelvic exenteration for tumor persistence |
| IIIa | 5000–6000 * | 2000–3000 or interstitial implant | — |
| IIIb | 5000–6000 * | 4000 (one application), 5000 (two applications) † | — |
| IVa | 6000 | 4000 (one application), 5000 (two applications) † | — |
| IVb | 500–1000 pulse two to four times 1 wk apart | Palliative | — |
* Patients with larger lesions or poor vaginal geometry merit the higher dose of external radiation.
† Two applications are suggested after whole-pelvis radiation with larger lesions or when the first application has less than optimum dosimetry.
Intensity-Modulated Whole Pelvic Radiotherapy
Traditional external-beam radiotherapy is based on field limits determined by bony landmarks, resulting in generous amounts of healthy tissue being encompassed by large treatment volumes. However, the margins of oncologic security are large, so target motion and the gross tumor volume (GTV) have a negligible impact. With increasing adoption of conformal treatment and attendant steep dose gradients, both the GTV and clinical target volume (CTV) must be precisely defined.
Using variable intensity across the face of the radiation beam to shape isodoses, (IMRT delivers a high tumor dose with minimization of radiation exposure to healthy tissue. Dosimetric advantages can be exploited using a combination of 3D planning and variable radiation intensity in each field. Reductions of both acute and chronic GI and genitourinary (GU) toxicity have been trumpeted among the dosimetric advantages of IMRT and some evidence suggesting that IMRT can spare bone marrow.
Uncertainty exists when considering IMRT for the management of locally advanced cervical cancer, including delineation of the required margins and what the acceptable degree of homogeneity is for the target. Importantly, given the large volume of hematopoietically active marrow in the pelvis and lower lumbar spine, in their review, Fernandez-Otz and Crook propose specific planning constraints be required, including use of a contouring atlas for pelvic volumes as currently used in current Radiation Therapy Oncology Group protocols (now part of NRG Oncology).
Radiation Therapy Oncology Group (RTOG) protocol 0418 established the current role of IMRT in gynecologic cancers to be relegated to the postoperative (ie, adjuvant) setting. The unacceptable toxicity of dose escalation to the pelvis and paraaortic regions using conventional radiotherapy has been prohibitive. However, with IMRT, simultaneous boosts to the target or affected lymph nodes results in an improvement of the therapeutic ratio and the added advantage of shortening the treatment time. Because of cervical-utero movement, the use of IMRT in the setting of the intact cervix requires continued investigation. RTOG protocols require a full bladder and empty rectum to minimize movement, but cervical tumor regression during treatment is also an issue, with a median volume reduction of 46% being reported in the literature. For this reason, Fernandez-Otz and Crook suggest that IMRT should be replanned during the final third of treatment to take advantage of the shrinking GTV.
Ly et al. developed dose-volume histograms of the target volume in a comparison of conventional radiotherapy, 3D conformal radiotherapy, and IMRT for cervical cancer. They reported that the planning target volume dose of the IMRT was significantly higher than the other two modalities and that when more than 30 Gy was administered in IMRT, organs at risk including the small intestine, rectum, bladder, and bone marrow received reduced volumes of radiations. To date, however, there are no head-to-head randomized phase III trials comparing IMRT with conventional radiotherapy in the primary treatment of locally advanced cervical cancer.
Radium and Cesium Therapy
Radium is the isotope that has been used traditionally in the treatment of cancer of the cervix. Its greatest value is that its half-life is approximately 1620 years; therefore, it provides a very stable, durable element for therapy. In recent years, both cesium and cobalt have been used for intracavitary therapy. Cesium has a half-life of 30 years; with the current technology, cesium provides an adequate substitute for radium. Four major technologies for the application of radium in the treatment of cervical cancer continue to be favored among gynecologists. Of these technologies, three are intracavitary techniques using specially designed applicators, and the fourth technique involves the application of radium in the form of needles directly into the tumor. The variations among the three techniques of intracavitary brachytherapy are found in the Stockholm, Paris, and Manchester schools of treatment ( Fig. 3.32 ). The differences are mainly found in the number and length of time of applications, the size and placement of the vaginal colpostats, and radium loading. In the United States, the tendency has been to use fixed radium applicators with the intrauterine tandem and vaginal colpostats originally attached to each other. Over the past 3 decades, a flexible afterloading system, Fletcher-Suit, has gained increasing popularity because it provides flexibility and the safety of afterloading techniques.
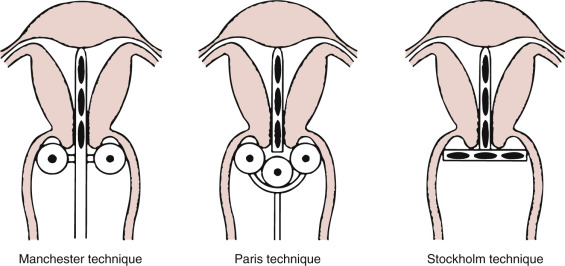
The Paris method originally used a daily insertion of 66.66 mg of radium divided equally between the uterus and the vagina. The radium remained in place for 12 to 14 hours, and the period of treatment varied from 5 to 7 days. An essential feature of the Paris method, and a part of the modification of this technique, is the vaginal colpostat, which consists of two hollow corks that serve as radium containers joined together by a steel spring that separates them into the lateral vaginal wall.
The Stockholm technique uses a tandem in the uterine cavity surrounded by a square radium plaque applied to the vaginal wall and portio vaginalis of the cervix. No radium is placed in the lower cervical canal, and vaginal sources are used to cover the cervical lesion. The uterine tandem and vaginal plaque are immobilized by packing and left in place for 12 to 36 hours. Two or three identical applications are made at weekly intervals.
The Manchester system is designed to yield constant isodose patterns regardless of the size of the uterus and vagina. The source placed in the neighborhood of the cervical canal is considered the unit strength. The remaining sources in the corpus and vagina are applied as multiples of this unit and are selected and arranged to produce equivalent isodose curves in each case and an optimal dose at preselected points in the pelvis. The applicator is shaped to allow an isodose curve that delivers radiation to the cervix in a uniform amount. The Fletcher-Suit system ( Fig. 3.33 ) previously mentioned is a variation of the Manchester technique.